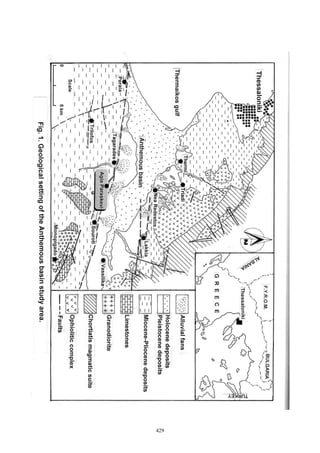
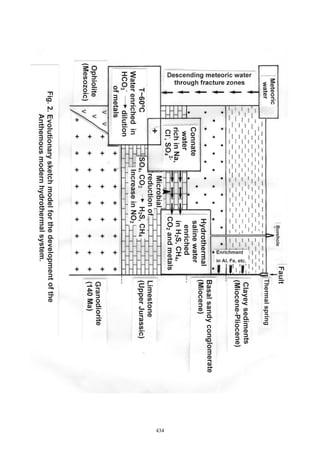
The document summarizes the geochemical conditions and environmental pollution from hydrothermal waters in the Anthemous basin in northern Greece. Key points:
- Hydrothermal springs discharge through faults, depositing metalliferous sediments and travertine rich in arsenic, iron, rare earth elements, and other metals.
- Spring waters have temperatures of 23-28°C, are saturated in sulfides, sodium, chloride, and bicarbonate, and enriched in pollutants like arsenic, manganese, and nitrites.
- Metalliferous sediments and travertine chemical deposits contain pyrite, chalcopyrite and are enriched in silicon, calcium, aluminum and various trace metals