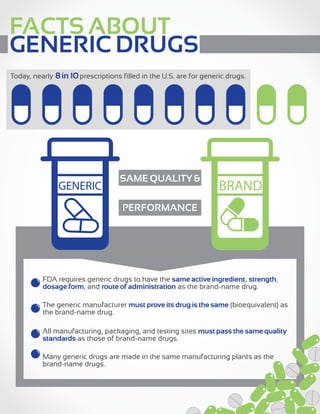
Nearly 8 in 10 prescriptions filled in the U.S. are for generic drugs. The FDA requires that generic drugs have the same active ingredients, strength, dosage form and administration as the brand name drugs. Generic manufacturers must prove their drugs are bioequivalent to the brand name drugs and meet the same quality standards in manufacturing. On average, generic drugs cost 80-85% less than their brand name counterparts and saved $158 billion in 2010 alone.