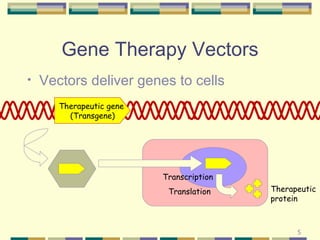
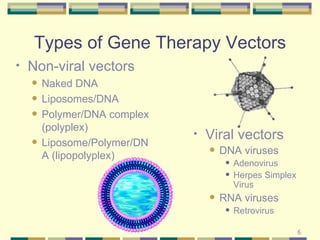
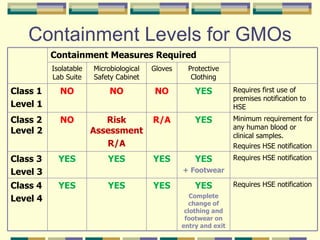
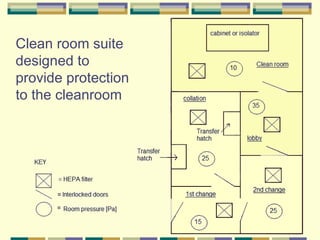
Gene therapy involves introducing genetic material into human cells to treat diseases. It aims to cure diseases by adding extra genes or replacing faulty genes. There are still many challenges to gene therapy, including developing safe and effective viral and non-viral vectors to deliver genes, avoiding unintended effects, and establishing regulations for clinical trials and production. As the field continues to advance, dedicated facilities and standardized procedures will be important to ensure patient safety.


















![Additional Information Gene Therapy Advisory Committee (GTAC) http://www. advisorybodies . doh . gov . uk /genetics/ gtac /index. htm Gene therapy trials worldwide. Provided by the Journal of gene medicine http://82.182.180.141/trials/index.html A guide to Genetically modified organisms (Contained Use) regulations 2000. Health and Safety Executive Genetically Modified Organism (Deliberate Release) Regulations 2002 [GMO(DR)]. Department for the Environment, Food and Rural Affairs (DEFRA) http://www.opsi.gov.uk/si/si2002/uksi_20022443_en.pdf Quality Assurance of Aseptic Preparation Services Fourth Edition. A.M. Beaney. Pharmaceutical Press 2006. Appendix 6. Gene Therapy. EU Clinical Trials Directive. http://www. wctn .org. uk /downloads/EU_Directive/Directive. pdf Implications of gene therapy for hospital pharmacists. Simpson.J, Stoner. N. www.pjonline.com/pdf/articles/ pj_20030726_ genetherapy .pdf](https://image.slidesharecdn.com/genetherapy-problemsandchallenges-090227130313-phpapp01/85/Gene-Therapy-25-320.jpg)
