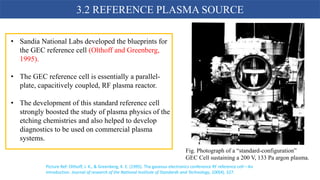
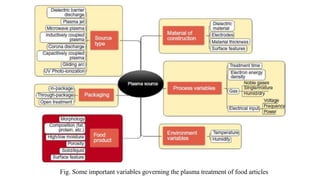
Cold plasma is a disruptive technology that can effectively decontaminate foods like strawberries while maintaining quality. Regulatory approval is a key challenge and involves establishing safety through various pathways depending on the country. The design of plasma machines for food processing requires an understanding of plasma chemistry and consideration of hygienic operation, analytics, and scalability. Standard reference plasma sources help advance research by allowing comparison of operating conditions and effects.