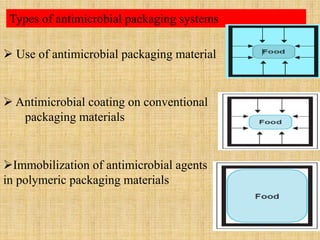
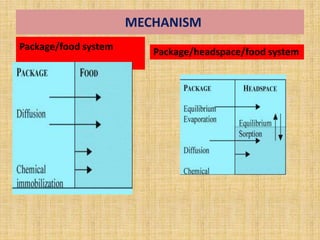
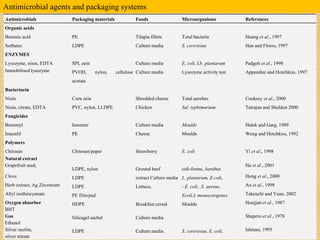
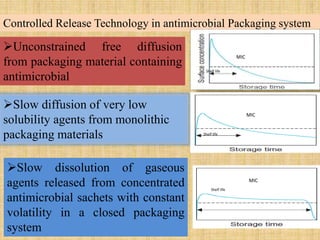
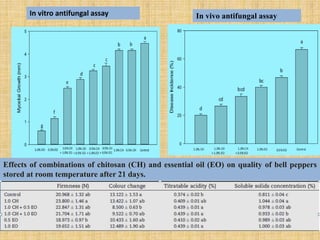
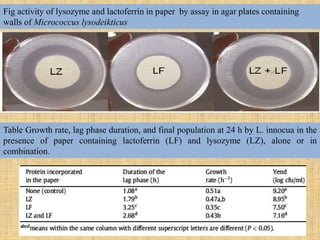
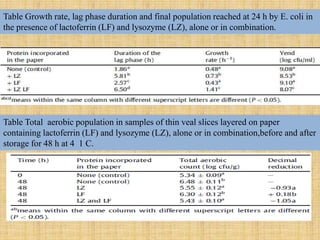
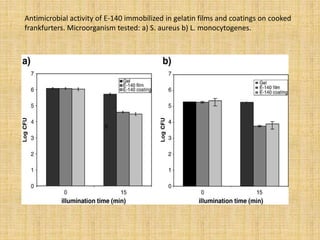
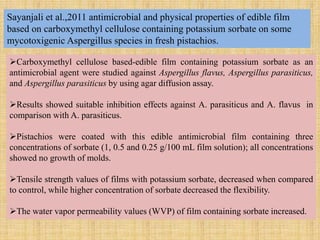
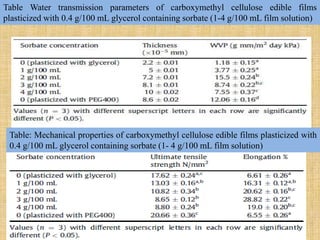
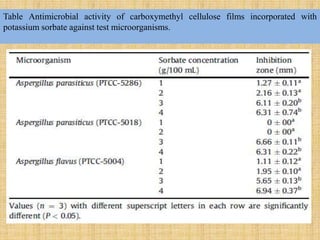
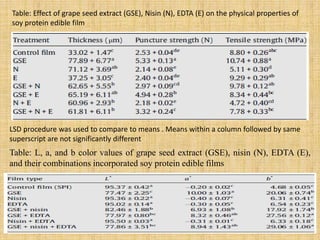
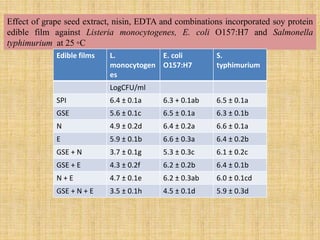
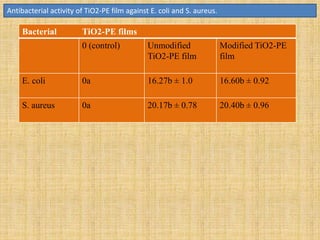
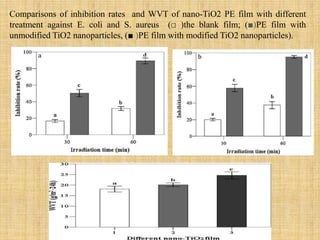
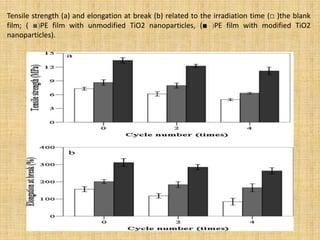
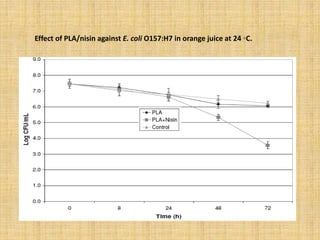
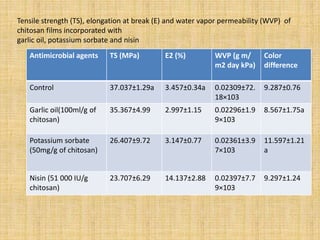
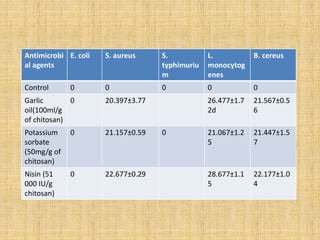
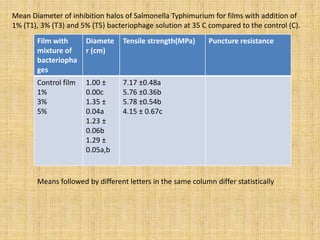
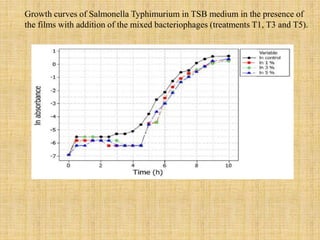
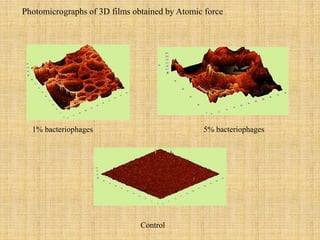
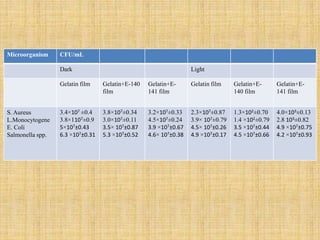
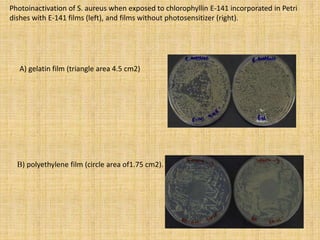
This document discusses antimicrobial packaging, a form of active packaging that uses antimicrobial agents to prevent the growth of microorganisms on food surfaces, thereby enhancing food safety and shelf life. Various types of antimicrobial packaging systems are described, including the use of coatings, sachets, and bio-based agents. The effects of different antimicrobial agents and packaging materials on food preservation are also highlighted, substantiated by various research studies.