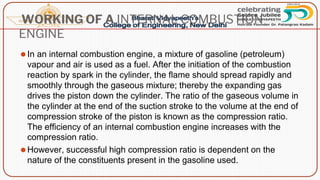
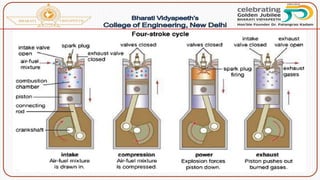
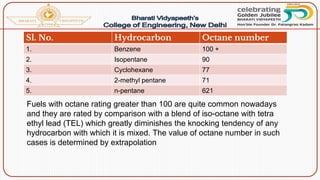
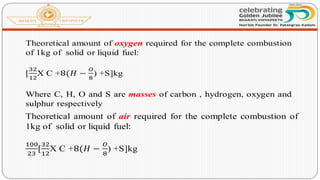
This document discusses the fundamentals of fuels, including their classification, calorific value, and analysis. It begins by defining what a fuel is and providing examples. It then covers:
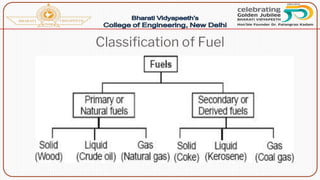
- Classification of fuels into primary/natural and secondary/derived types.
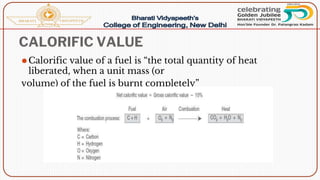
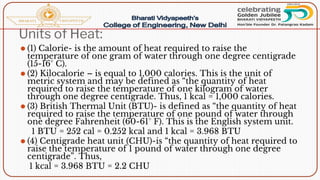
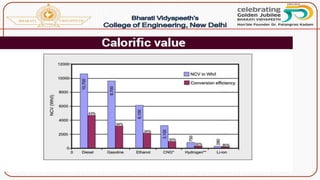
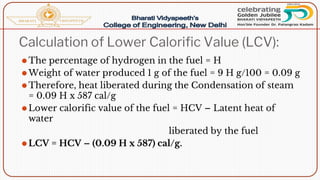
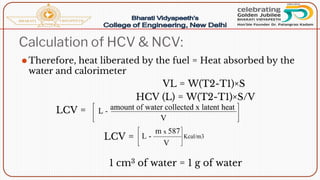
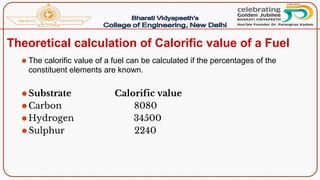
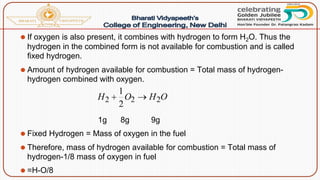
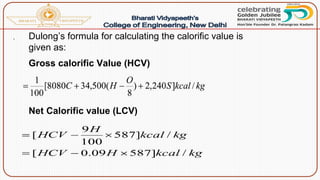
- Calorific value and different units used to measure heat such as calories, kilocalories, BTUs. It defines gross/higher and lower/net calorific values.
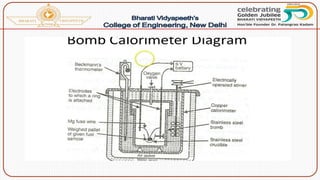
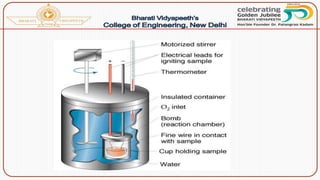
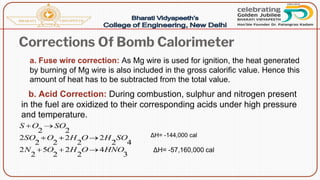
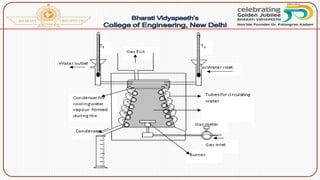
- Methods of determining calorific value using a bomb calorimeter, involving burning a fuel sample and calculating the heat released based on temperature changes.
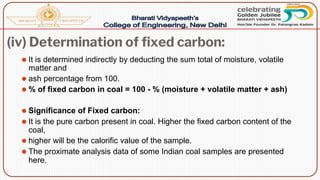
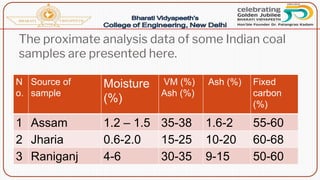
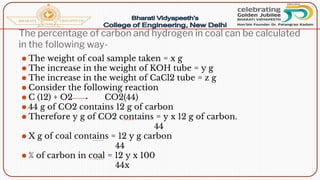
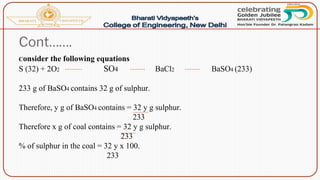
- Proximate analysis of coal to determine moisture, volatile matter, ash, and fixed carbon content.


















































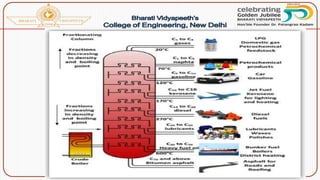




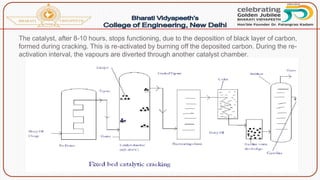
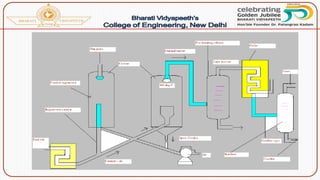
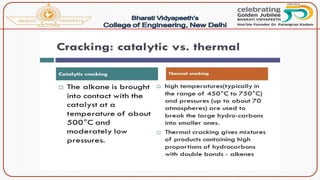
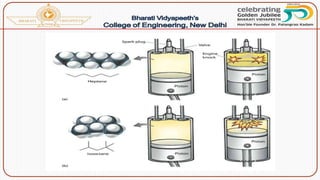
![Catalytic cracking:
The quality and yield of gasoline produced by cracking can be
greatly improved by using a suitable catalyst like aluminium silicate
[Al2(SiO3)3] or alumina [Al2O3]. There are two methods of catalytic
cracking in use:
(a)Fixed-bed catalytic cracking:
(b) Moving-bed catalytic cracking:](https://image.slidesharecdn.com/fuels-230503143622-96fe8dc5/85/Fuels-pptx-75-320.jpg)