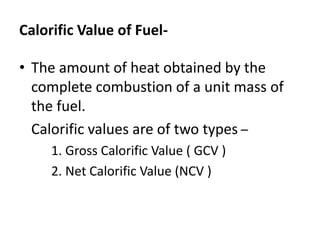
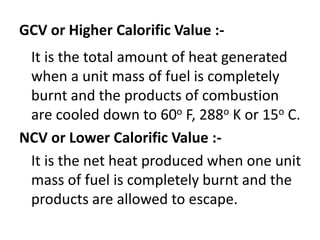
This document defines fuels and classifies them as primary or secondary. It discusses the characteristics of good fuels and defines gross calorific value and net calorific value. It describes how to determine calorific values using bomb calorimetry and gas calorimetry through calculations involving the mass of fuel burned, water mass, temperature changes, and corrections. Specific solid, liquid and gaseous fuels are also listed along with the combustion of fuels.





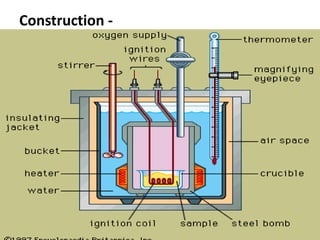




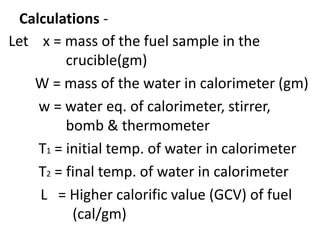
![Heat liberated by the burning of fuel = x L cal
Heat absorbed by water = W x (T2-T1)
Heat absorbedby apparatus (calorimeter) =
w x (T2-T1 )
Hence total heat absorbed/gained by water,
apparatus etc = W x (T2-T1 ) + w x (T2-T1 ) cal
= [(W + w) (T2-T1 )] cal .
Heat liberated by the fuel = Heat gained by
the water & calorimeter](https://image.slidesharecdn.com/fuelppt-240226162131-77fe3e52/85/Calorific-value-measurement-in-engineering-ppt-18-320.jpg)
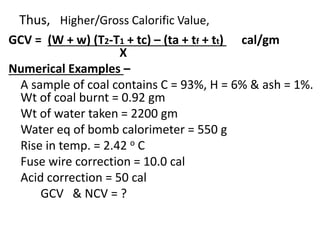
![Therefore X L = [(W + w) (T2-T1 )]
L = (W + w) (T2-T1 ) cal/gm
X
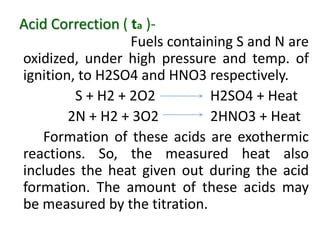
Corrections –
Fuse Wire Correction ( tf )–
The heat liberated, also
include the heat given out by the ignition of
fuse wire used. Hence it must be subtracted
from the total value.](https://image.slidesharecdn.com/fuelppt-240226162131-77fe3e52/85/Calorific-value-measurement-in-engineering-ppt-19-320.jpg)