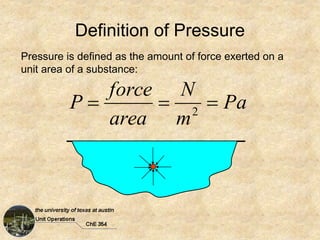
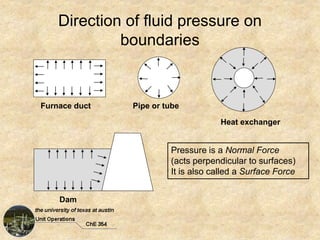
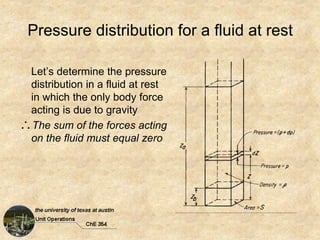
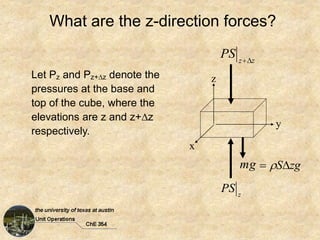
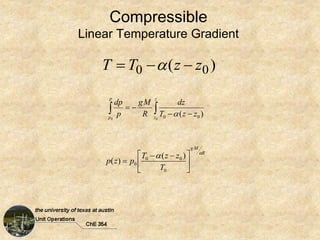
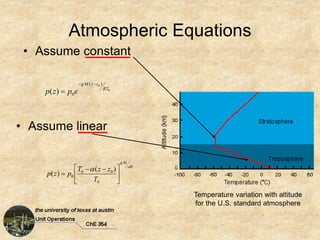
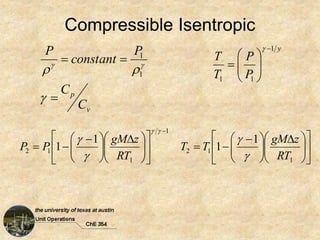
Okay, here are the steps to solve this problem:
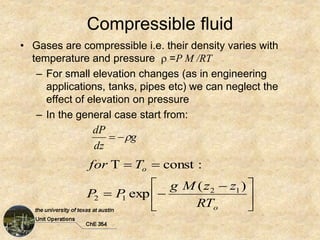
1) Given: T0 = 15°C, P0 = 760 mm Hg = 101.325 kPa, dz = 1000 m
2) Temperature variation with elevation: dT/dz = -5°C/1000 m
3) Use ideal gas law: P1V1/T1 = P2V2/T2
4) Substitute values and solve for P2: P2 = P0(T0/T2) = 101.325(15/(15 - 5(8.847))) = 33.8 kPa
So the air pressure on top of Mt. Everest at 8847 m is 33.