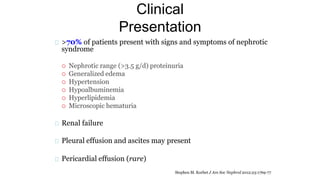
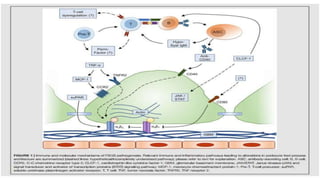
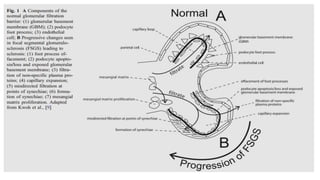
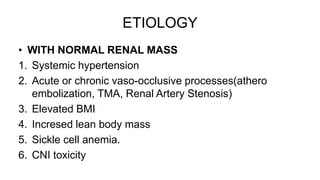
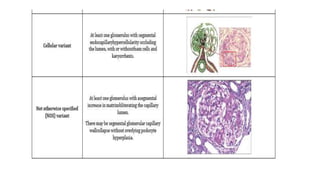
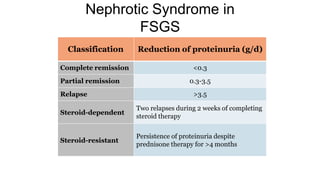
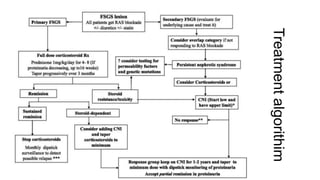
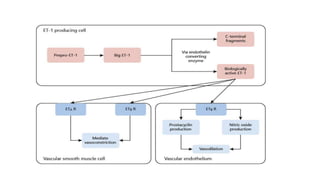
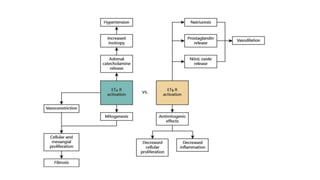
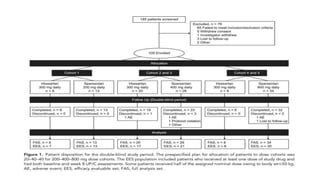
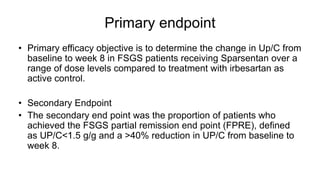
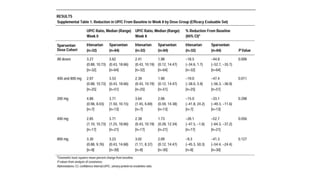
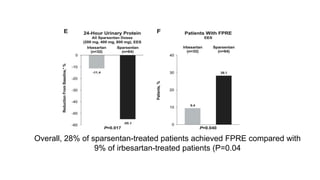
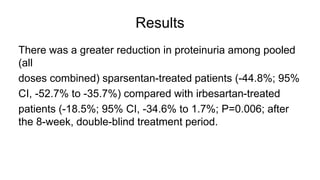
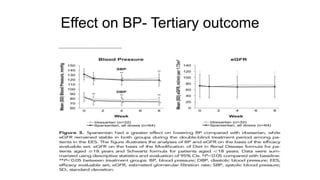
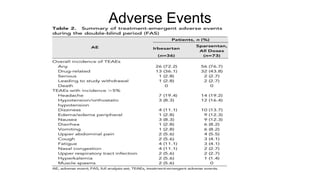
Sparsentan, an endothelin receptor antagonist, reduced proteinuria more than irbesartan, an angiotensin receptor blocker, in patients with focal segmental glomerulosclerosis (FSGS) in an 8-week clinical trial. 28% of patients on sparsentan achieved a partial remission compared to 9% on irbesartan. Sparsentan also lowered blood pressure similarly to irbesartan with adverse effects being mild. However, longer term studies are still needed to determine if sparsentan preserves kidney function in patients with FSGS.