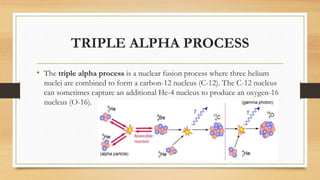
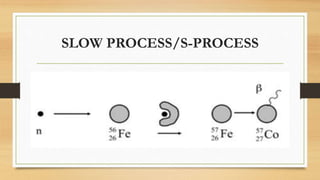
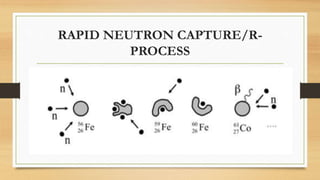
The document discusses the synthesis of new elements, beginning with the earliest elements of hydrogen and helium formed in the Big Bang. It then explains how elements up to iron are formed through nuclear fusion in stars, and how heavier elements are created through neutron capture processes during supernovae or in the cores of massive stars. The text also outlines several key discoveries in the development of the periodic table and synthesis of both naturally occurring and man-made transuranic elements.