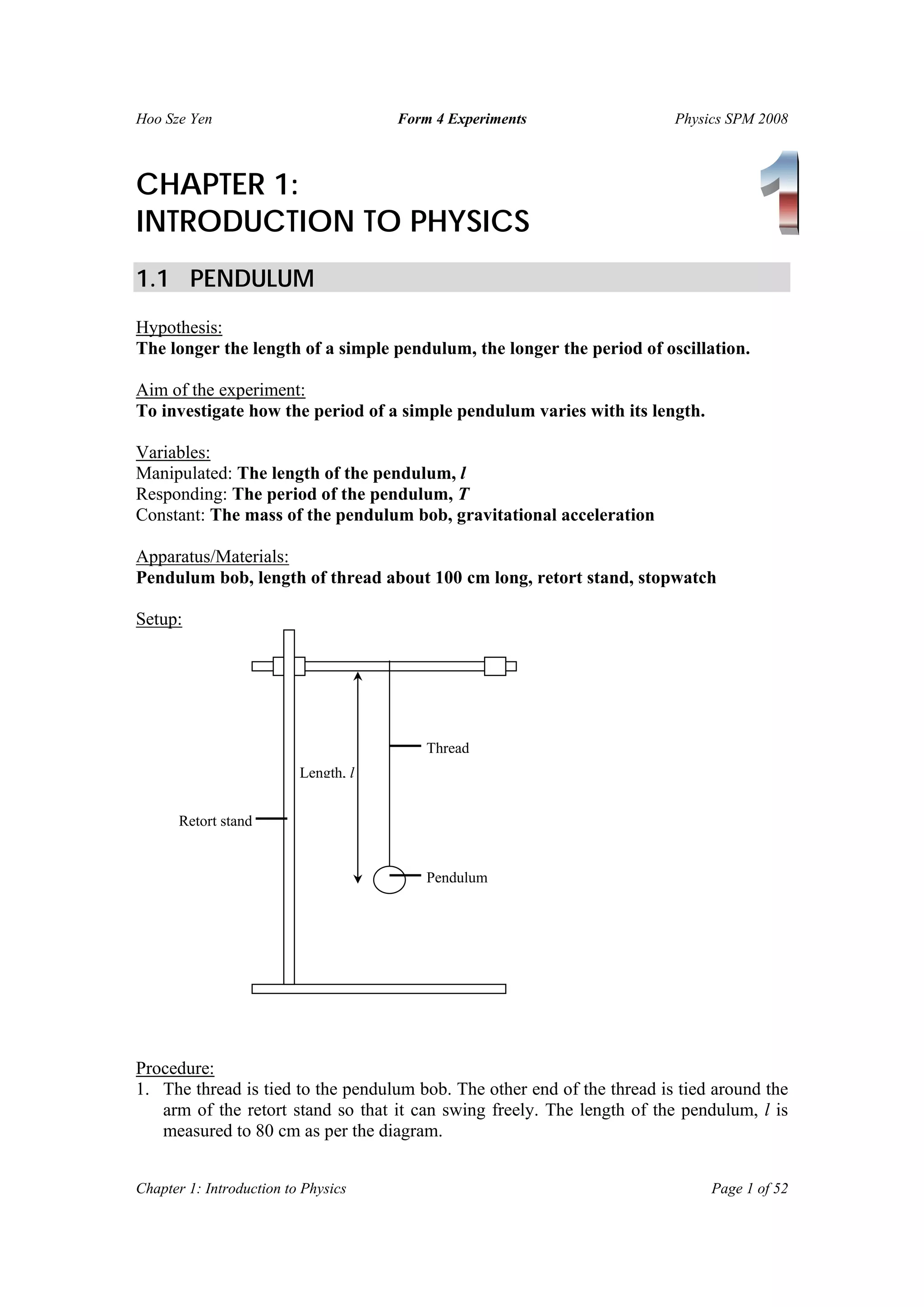
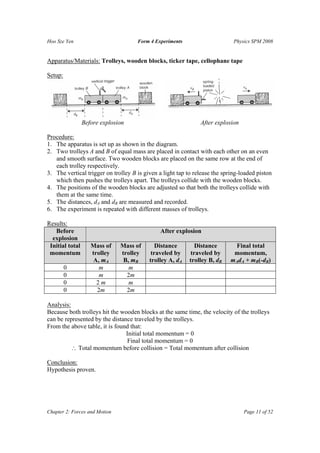
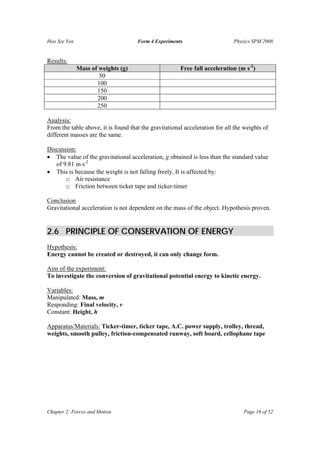
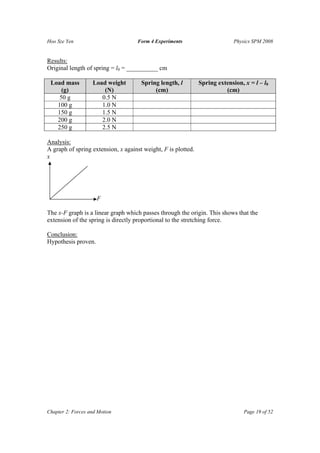
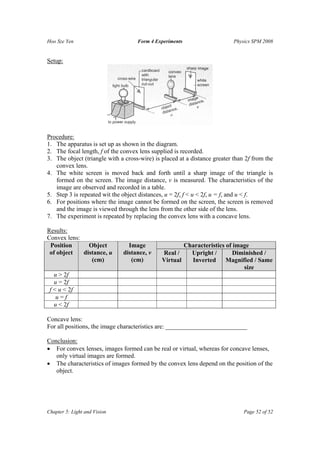
The document summarizes several physics experiments on topics like simple pendulums, inclined planes, inertia, and conservation of momentum. It includes the hypotheses, variables, procedures, results and conclusions for each experiment. The experiments are designed to investigate relationships between various physical quantities like the period of a pendulum varying with its length, the velocity of an object on an inclined plane relating to the angle of incline, and momentum being conserved before and after collisions.