This document presents the forensic pharmacy syllabus as per the PharmD curriculum for 2020-2025, detailing the role of forensic pharmacists in various legal contexts and the historical development of pharmacy education in Pakistan. It covers relevant legislation, national health policies, the importance of essential drugs, and the ethical considerations in the pharmaceutical industry. Additionally, it highlights the involvement of pharmacists in areas such as drug regulation, malpractice, and public health issues.


























![13
13
Provided that the exemptions under Sections 132 and 133 of the Code of
Civil Procedure, 1908 (Act V of 1908), shall be applicable to requisitions for
attendance under this clause;
(h) lock and seal any factory, laboratory, shop, building, store-house or godown,
or a part thereof, where any drug is or is being manufactured, stored, sold or
exhibited for sale in contravention of any of the provisions of this Act or the
rules;
(i) forbid for a reasonable period, not exceeding two weeks or such further
period, which shall not be more than three months, as the Inspector may, with
the approval of the Provincial Quality Control Board, the Central Licensing
Board, the Registration Board, or the licensing authority, as the case may be,
specify, any person in charge of any premises from removing or dispensing of
any drug, article or other thing likely to be used in evidence of the
commission of an offence under this Act or the rules; and
(j) exercise such other powers as may be necessary for carrying out the purposes
of this Act or any rules:
Provided that the powers under causes (f) to (j) shall be exercisable only
by an Inspector specifically authorized in this behalf, by an order in writing,
by the Government appointing him, subject to such conditions as may be
specified in such order.
[Second proviso omitted]
(2) The provisions of the Code of Criminal Procedure, 1898 (Act V of 1898), in
so far as they are not inconsistent with the provisions of this Act, shall apply to searches
and seizures made under this Act.
19. Procedure for Inspectors.- (1) Where an Inspector seizes any drug or any
other article under section 18, he shall tender a receipt therefore in the prescribed form.
(2) Where an Inspector takes a sample of a drug for the purpose of test or
analysis, he shall intimate such purpose in writing in the prescribed form to the person
from whom he takes it and, in the presence of such person unless he willfully absents
himself, shall divide the sample into five portions and effectively seal and suitably mark
the same and permit such persons to add his own seal, if any, and mark to all or any of
the portions so sealed and marked:
Provided that, where the sample is taken from premises whereon the drug is being
manufactured, it shall be necessary to divide the sample into three portions only:
Provided further that, where the drug is made up in containers of small volume,
instead of dividing a sample as aforesaid, the Inspector may, and if the drug be such that
it is likely to deteriorate or be otherwise damaged by exposure shall, take three or four, as](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-27-320.jpg)




















![(d) one pharmacist, to be nominated by the Federal Government;
(e) one medical specialist from the Army Medical Corps. to be nominated by the Federal Government.
(f) one pharmaceutical chemist or expert in quality control, to be nominated by the Federal
Government;
(g) the Drugs Controller, Ministry of Health, Government of Pakistan who shall be its ex-officio
Secretary;
(h) one representative, not below the status of an officer of BBPS- 19 [.....], of each of the Ministries of
Commerce Industries & Justice to be nominated by the Federal Government; and
(i) one representative of the Central Board of Revenue, not below the status of an officer of B-20, to
be nominated by the Federal Government;
(j) Cost Accountant of the Ministry of Health;
(k) One physician, to be nominated by the Federal Government;
(j) One Surgeon, to be nominated by the Federal Government. or an officer of the Provincial Health
Department not below the status of Additional Secretary, to be nominated by the Secretary, Health
Department of that Province. and
(m) one expert in veterinary medicine to be nominated by the Federal Government.
(2) No person who is a member of the Appellate Board shall be nominated to the Central Licensing
Board.
(3) The members of the Central licensing Board, other than its ex officio members, shall hold office for
three years and shall be eligible for renomination.
(4) The Central Licensing Board may co-opt any other person who is expert in the pharmaceutical or
medical profession for advice on any particular matter under consideration.
(5) The meetings of the Central Licensing Board may be held at such time as the Board may deem fit
and, on the request of any of its members, the Chairman may at any time call a meeting if there is any
important matter for its consideration.
(6) In the absence of the Chairman, the Board may elect one of its members to preside over a
meeting.
(6-A) The quorum to constitute a meeting of the Board shall be one third of its total membership.
(7) The Central Licensing Board may authorise the Chairman to any of its members to perform any
specific function of the Board for a specified period.
(8) The Central Licensing Board shall follow such policy directing as the Federal Government may
issue from time to time.
(9) No act or proceeding of the Central Licensing Board shall be invalid merely on the ground of the
existence of any vacancy in, or any defect in the constitution of the Board.
(10) The chairman and the Secretary of the Central Licensing Board shall, after the Board has
approved the issuance of a licence sign the licence.
(11) Subject to rule 14, the Central Licensing Board may appoint a licensing authority or authorities for
such purpose as it may deem fit.
9. Powers of the Central Licensing Board: (1) The members of the Central Licensing Board shall
exercise all the powers of an Inspector without restriction as to area, and shall have the powers of a
Provincial Inspector in relation to Section 30.
(2) In the exercise of their powers the members of the Central Licensing Board shall follow the](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-48-320.jpg)



![of drugs included in Schedule C, permit the manufacture of such drugs under the active direction and
personal supervision or a person holding a degree in medicine or veterinary sciences of a university in
Pakistan or any other institution recognised by the Federal Government, with at least three years
experience in the manufacture, testing and analysis of biological products which are intended to be
produced:
Provided further that the Central Licensing Board, may, in the case of anufacture of disinfectant fluids,
insecticides liquid paraffin, medicinal gases, non-chemical contraceptives, plaster of paris, surgical
dressing or chemicals for the manufacture of which the knowledge of pharmacy or pharmaceutical
chemistry is not essential, permit manufacture of the drug under the active direction and personal
supervision of competent staff who, […..] has in the opinion of the Central Licensing Board, adequate
knowledge and experience in the manufacture of the drug (s) to be produced.
(d) The applicant shall establish an independent Quality Control Department and maintain separate
staff, premises and adequate laboratory equipment for carrying out tests of strength, quality and purity
of the substances being or to be used in the manufacture.
Provided further that a person already approved by the Central Licensing Board as the production
incharge of a pharmaceutical firm shall continue to be the technical supervisor of that firm for the
purposes of this rule.
(e) The Quality Control Department shall be independent of the manufacturing unit and its incharge
shall be whole time employee of the manufacturer and shall possess a degree in pharmacy, or a
degree in science with chemistry or a degree in medicine or pharmacology (for pharmacological
testing) or a degree in microbiology (for microbiological testing) and has sufficient experience in
testing of drugs:
Provided that in the case of drugs specified in Schedule C, the Central Licensing Board may allow the
applicant to make arrangements with some other institution approved by the Central Licensing Board
for such tests to be regularly carried out on his behalf by that institution.
17. Licence to manufacture drugs by way of repacking: (1) A licence to manufacture drugs by way
of repacking is required for the repacking of such drugs, and under such conditions, as are specified
in Schedule D.
(2) Where a person possesses or applies for a licence to manufacture by way of formulation and he
also intends to conduct repacking of drugs, he may conduct such repacking under the same licence
subject to the approval of, and under such conditions as, the Central Licensing Board may specify.
18. Condition for the grant or renewal of a licence to manufacture drugs by way of repacking:
Before a licence to manufacture drugs by way of repacking is granted or renewed, the Central
Licensing Board shall satisfy itself that the following conditions are complied with by the applicant,
namely :--
(a) adequate space and equipment shall be provided;
(b) repacking operation shall be carried out under hgygienic conditions and under supervision of
technical staff provided for in clause (c) of rule 16;
(c) adequate arrangements shall be provided for carrying out the tests for strength potency, quality
and purity of the drugs to be repacked.
19. Conditions of licence to manufacture, by way of basic manufacture, semi-basic
manufacture formulation and repacking of drugs: (1) A licence to manufacture by way of basic,
semi-basic manufacture, formulation or repacking of drugs shall be subject to the conditions stated
herein, if any, and to the further condition that the licensee shall continue to maintain conditions on the
basis of which he was granted a licence.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-52-320.jpg)





![made within sixty days after the expiry of the registration and when an application has been made as
aforesaid the registration shall subject to the orders passed on the application for the renewal
continue in force for the next period of five years :
Provided further that, if in the opinion of the Registration Board it is necessary so to do in the Public
interest, it may provisionally register a [....] drug for period of two years.
28. Certificate of registration: A certificate of registration of drug shall be issued in Form 6.
29. Procedure for registration: (1) The Registration Board may, if it considers necessary, cause the
application for registration and the information and material supplied to it under rule 26 to be
evaluated by a Committee on Drugs Evaluation consisting of experts related to the aspect of the drug
to be evaluated and obtain its report.
(2) The Registration (2) The Registration Board may, before issuing a registration], cause the
premises in which the manufacture is proposed to be conducted to be inspected by itself or by its sub-
committee or by a panel of Inspectors or experts appointed by it for the purpose, which may examine
all portions of the premises and the plant and appliances, inspect the process of manufacture
intended to be employed and the means to be employed for standardising, if necessary, and testing
the substances to be manufactured and enquire into the professional qualifications of the technical
staff employed.
(3) Where inspection under sub-rule (2) is carried out by a Sub-Committee or panel of experts or
Inspectors appointed under the said sub-rule, it shall forward to the Registration Board a detailed
report of ;he result of the inspection.
(4) If the Registration Board, after such further enquiry, if any, as it may consider necessary, is
satisfied of its safety, efficacy, quality and economical value or where the public interest so requires, it
may register the drug and issue a certificate of registration in Form 6, subject to such specific
conditions as it may specify.'
(5) The Registration Board may, while registering a drug under sub-rule (4), approve the details as
supplied by the applicant or approve them with amendments as it may deem fit in respect of the
following particulars, namely :--
(a) the name under which the drug may be sold;
(b) the labelling;
(c) the statement of all the representations to be made for the promotion of the drug in respect of--
(i) the claims to be made for the drug;
(ii) the route of administration;
(iii) the dosage;
(iv) the contra-indications, the side effects and precautions if any; and
(d) Omitted by S.R.O. 551(1)//93, dated 3. 7. 1993.
(5-A) Where the Registration Board registers a new drug, it may recommend to the Federal
Government for fixation of maximum price of such drug.
(6) The Registration Board shall, before registering a new drug for which the research work has been
conducted in other countries and its efficacy, safety and quality has been established therein, require
the investigation on such pharmaceutical, pharmacological and other aspects, to be conducted and
clinical trials to be made as are necessary to establish its quality and, where applicable, the biological,
availability, and its safety and efficacy to be established under the local conditions:
Provided that under special circumstances to be recorded in writing, the Registration Board may](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-58-320.jpg)
![register a drug and require such investigations and clinical trials to be conducted after its registration.
(7) A new drug, where new method of manufacture is contemplated or a change is proposed in
source, standard or specification of the active ingredient or the finished product, may not require full
investigations and clinical trials except in so far as they are necessary for the purpose of establishing
bio-equivalence, absorption, acceptability or other such features.
(8) Where it is necessary in the public interest so to do, the Registration Board may register a drug on
its own motion without having received any application for registration.
(9) If the Registration Board is not satisfied as to the safety, efficacy, quality or economic value of a
drug, or where the public interest so requires it may, [ .]..., reject the application for registration and
inform the applicant of the reasons for such rejection in writing.
(10) Rejection of an application for the registration of a drug shall not debar an applicant from
submitting a fresh application under rule 26.
30. Conditions or registration of drug: (1) The relevant provisions of the Ordinance and the rules in
respect of the registered drug, shall be complied with.
(2) The import, manufacture and sale of drugs shall be in accordance with the information contained
in the applications in respect of those drugs or in any supplementary information or, where such
information was amended by the Registration Board, in accordance with such amended information
on the basis of which such drugs were registered:
Provided that deviations from any such information may be made only after obtaining prior approval of
the Registration Board.
(3) The indications, contra-indication, side effects, the dosage and cautions, if any, as have been
approved for the purpose of registration of a drug shall be clearly specified in the labelling and
promotion.
(4) Every drug shall be produced in sufficient quantity so as to ensure its regular and adequate supply
in the market.
(5) The manufacture of any drug shall not, without the prior approval of the Registration Board, be
discontinued for period which may result in its shortage:
Provided that in the circumstances beyond the control of a manufacturer,, of a drug which may lead to
reduction in the production of that drug, the circumstances may be intimated to the Registration
Board.
(6) A record of quarterly production and disposal of a drug shall be maintained and supplied to the
Chairman of the Registration Board in Form 7 in the months of January, April, July and October each
year.
(7) In case of an imported drug, the indenter or any other approved representative in Pakistan of the
foreign firm shall ensure regular and adequate supply of tee drug in Pakistan.
(7-A) The indenter, importer or manufacturer's authorised agent shall issue a warranty in Form 2-A for
any drug indented or sold by him for the purpose of re-sale or distribution; and
(8) In respect of new drug, records, including adequately organised and indexed files, shall be
maintained containing full information regarding--](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-59-320.jpg)




![SCHEDULE A
[See rule 2 (e)]
Form 1
[See rule 5 (/)]
APPLICATION FORM GRANT OF A LICENCE TO MANUFACTURE BY WAY OF
FORMULATION/BASIC MANUFACTURE/SEMI-BASIC MANUFACTURE/REPACKING
I/We ...........of .........hereby apply for the grant of a licence to manufacture by way of........................on
premises situated at ...................
2. The drug(s) or class(es) of drugs intended to be manufactured :-
(1) Class(es) of drugs.
(2) Dosage form(s) of drugs.
(3) Name of the drug(s).
3. I enclose :-
(i) Particulars regarding the legal status of the applicant (i.e. in case of proprietorship the names) of
proprietors and their address (es), in the case of firm the name and names and addresses of its
partners and in the case of company the name and address of the company and its directors).
(ii) Details of the premises including layout plan of the factory.
(iii) Details of the section-wise equipment and machinery for manufacture and quality control.
(iv) Names and qualifications of the Production Incharge and Quality Control Incharge for supervising
manufacturing processes and Quality Control Department, and other technical staff working in these
departments.
4. The premises and plan will be ready for inspectionon or are ready for inspection.
Dated.................. Signed……………………
Place.................... Name, designation and address ........................
-----------------------](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-64-320.jpg)

![FORM 1-A
[See rule (5(I)]
APPLICATION FORM FOR RENEWAL OF A LICENCE TO MANUFACTURE DURGS BY WAY OF
FORMULATION/BASIC MANUFACTURE/SEMI-BA SIC MANUFACTURE/REPACKING
I/We ............................ of ........................ hereby apply for the renewal of a licence to manufacture by
way of on premises situated at ....................................
2. The drug(s) or class(es)of drugs intended to be continued to be manufactured:-
(i) Class(es) of drugs.
(ii) Dossage form(s) of drugs.
(iii) Name of the drug(s) registered/approved.
3. There have been/have not been any change in respect of
(i) Name of the proprietor/directors/partner(s)
(ii) Details of the premises including layout plan of the factory.
(iii) Details of the section-wise equipment and machinery for manufacture and quality control.
(iv) Names and qualifications of the Production Incharge and Quality Control Incharge for supervision
of manufacturing processes and Quality Control Departments, and other technical staff working in
these departments
4. Statement of the Central Research Fund.
Attested copies of the last two income tax assessment orders of the Income Tax Department
attached.
Following statement, as per audited accounts/based on Income Tax Return for the last five years:-
Year Investment Turn-over CRF due C R F paid as per Col. 4
1 2 3 4 5
Date Signed………………………….
Place Name, designation and address
of the signatory .......................
Note:-Strike off which is not applicable
----------------------](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-66-320.jpg)
![FORM 2
[See rule 7] GOVERNMENT OF PAKISTAN
Licence to Manufacture
is/are hereby licensed to manufacture by way of Basic Manufacture/Semi Basic
manufacture/Formulation/Repacking at the following premises:-
2. This licence permits the manufacture of
3. This licence shall, in addition to the conditions specified in the rules made under the Drugs
Ordinance/Act, 1976, be subject to the following conditions namely:-
(i) The licence will be in force for a period of five years from the date of issue unless earlier
suspended or cancelled.
(ii) The licence authorises the sale by way of wholesale dealing and storage for sale by the licensee of
the products manufactured under this licence, subject to the conditions applicable to licences for sale.
(iii) Name of the approved expert staff.
.......................................
.......................................
.......................................
.......................................
Date of issue .................
Secretary, Central Licensing
Board.
(Seal) Chairman, Central Licensing
Board.
FORM 2A
(See rules 19 and 30)
Warranty under Section 23(I)(i) of the Drugs Act, 1976
I...................being a person resident in Pakistan, carrying on business at (full address) ..................
under the name of.....................(and being an importer/indenter/authorised agent of ..................), do
hereby give this warranty that the drugs here-under described as sold/indented by me/specified and
contained in the bill of sale, invoice, bill of lading or other document describing the goods referred to
herein do not contravene in any way the provisions of section 23 of the Drugs Act, 19.76.
Dated (Signed)
1. Name(s)· of the drug(s):
(i)
(ii)
Batch number(s)
2. Description of bill of sale, invoice, bill of lading or other document (if any).](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-67-320.jpg)
![Signed ..............................
------------------------
FORM 3
[See rule 21(I)]
APPLICATION FOR LlCENCE TO MANUFACTURE DRUG(S) FOR EXPERIMENTAL PURPOSES.
I/We ............... of ........... hereby apply for a licence to manufacture drug(s) specified below for
experimental purposes at .......................... and I/We undertake to comply with the conditions
applicable to the licence under rule 22 of the Drugs (Licensing, Registering and Advertising) Rules,
1976.
1. Name and quantity of drug(s) to be manufactured for the said purposes:.
Signature............................
Name ..............................
Address ...........................
Countersigned by .......................
FORM 4
[See rule 21(3)]
LICENCE TO MANUFACTURE DRUG(S)
FOR EXPERIMENTAL PURPOSES
Mr./Messrs .................... of .................. is/are hereby licensed to manufacture the drug(s) specified
below for experimental purposes at ........................... :. or at such other place(s) at the. Central
Licensing Board may from time to time permit.
2. The licence is subject to the conditions prescribed in rule 22 of the Drugs (Licensing, Registering
and Advertising) Rules, 1976, and such other conditions as n3ay be subsequently prescribed or
Specified by the Central Licensing Board in this behalf.
3. This licence shall unless previously suspended or cancelled be in force for a period of two years
from the date specified below:-
Name of drugs with quantity to be manufactured.
Date:.................
Place:.................
Licensing Authority.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-68-320.jpg)
![FORM 5
[See rule 26(I)]
APPLICATION FORM FOR REGISTRATION OF A DRUG FOR LOCAL MANUFACTURE
I/we.........................of ................hereby apply for registration of the drug namely ..................details of
which are enclosed.
Date .......................
Place ......................
ENCLOSURE OF THE APPLICATION FOR REGISTRATION OF A DRUG
1. Name and address of the manufacturer ·
2, Name of drug ·
(a) Generic/international non-proprietory name:
(b) Proprietory name, if any:
3 Name under which drug is proposed to be sold
4. Dosage from of the drug:
5. Composition of the drug, stating quantity of each active and non-active ingredient(s) per
unit or as a percent age of total formulation :
6. Proposed dosage :
(a) for adults.
(b) children by age group.
(c) infant
(d) special groups.
7. Main Pharmacological group to which the drug belongs:
8. Pharmacological and clinical data :
(a) recommended clinical use and the claims to be made for the drug.
(b) contra-indications.
(c) toxicity or the side-effects.
(d) any directions for the use to be included in the labelling, warning and precautions in use :
symptoms of over dosage should be given alongwith the treatment including antidotes, where
required.
9. Proposed route of administration.
10. Description of the method of manufacture and quality control with details of the
equipment.
11. Specifications, with details of analytical procedure for each ingredient and the finished
drugs (not required in case of a drug for which pharmacopocial standards recognised under
the Drugs Act, 1976, are claimed).](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-69-320.jpg)

![FORM -5(A)
[See rule 26 (1)]
APPLICATION FORM FOR REGISTRATION OF AN IMPORTED DRUG
I/We ........................of ..........................hereby apply for registration of the drug,
namely....................details of which are enclosed.
Date .......................
Place ......................
Signed...........................
ENCLOSURES OF THE APPLICATION FOR REGISTRATION OF A DRUG
1. Name, address and status of the applicant:
2. Name and address of the manufacturer:
3. Name of the drug:
(a) Generic international non-proprietory name:
(b) Proprietory name, if any:
4. Name of drug, under which it is proposed to be sod:
5. Dosage form of the drug:
6. Composition of the drug stating quantity of each active and non-active ingredients per unit dose or
percentage of total formulation:
7. Proposed dosage:
(a) for adults.
(b) children by age group.
(c) infants.
(d) special groups,
8. Main Pharmacological group to which the drug belongs:
9. Proposed route of administration:
10. Pharmacological and clinical data :
(a) recommended clinical use and the claim to be made for the drug.
(b) contra-indications.
(c) toxicity or the side-effects.
(d) any directions for. use to be included in the labelling warnings and precautions in use: symptoms
of overdosage should be given alongwith the treatment including antidotes where required.
11. Specifications with details of analytical procedure (not required in case of a drug for which the
pharmacopocial standards recognised under the Drugs Act, 1976 are claimed):
12. Bio-availability studies:
13. Stability studies :
14. Proposed shelf life with storage conditions, if any :](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-71-320.jpg)
![15 Type of container :
16. Labelling : (Specimen to be enclosed alongwith a .sample and undertaking to refrain from
counterfeiting shall also be submitted) :
17. Proposed C and F and maximum retail price (in case of imported drug) :
18. Justification :
19. Certificate regarding sale and G.M.P. in the country of origin (in English and in Form 5 (c) :
20. Certificate of registration by F.D.A. of USA. Committee on Safety of Medicines of U.K. or
corresponding agencies of France, West Germany, Japan, Sweden. and Denmark.
21. Patent number, if any, with date and its date of expiry :
22. Undertaking to manufacture drug locally within two years. If it is not possible, the reasons therefor.
FORM-5B
[See rule 26(3A)]
APPLICATION FORM FOR RENEWAL OF REGISTRATION OF ALL KINDS OF DRUGS
I/We .................... of ................. hereby apply for renewal of registration of the drug, namely
.................details of which are as follows ·
1. Name and address of the manufacturer:
2. Name and address of the agent or indentor in case of imported drug -
3. Whether the drug is registered for local manufacture or import ·
4. Name of the registered drug, with its registration number and date or initial ,registration and last
renewal '
5. Changes, if any, in information furnished at the time of initial registration or last renewal
6. If withdrawn from the market anywhere ·
(i) Country.
(ii) Reasons thereof.
Place..................... Signature ..................
Date....................... Name, and address of the
signatory ............................](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-72-320.jpg)
![FORM-5C
TO WHOM IT MAY CONCERN CERTIFICATE OF DRUGS REGISTERED UNDER
THE DRUGS ACT, 1976
Name and dosage form of product .........................................
Name and amount of each active ingredient
.............................................................................................
Manufacturer and or when applicable the person responsible for Placing the Product on the market
............................ Address(es)......................................................................
It is certified :
* This product has been authorised to be place of the market for use in this country.
*Number of Registration and date of issue if plicable.
*This product has not been authorised to be placed on the market for use in this country for the
following reason-
..........................................................................................................................
..........................................................................................................................
..................................................................................
........................................
It is also certified that (a) the manufacturing plant in which the product is produced is subject in
inspections at suitable intervals, and (b) the manufacturer conforms to requirements for good
practices in the manufacture and quality control, in respect of products to be sold or distributed within
the country of origin or to be exported.
(Signature of designated authority (Place and date)
FORM 6
[See rules 28 and 29(4)]
GOVERNMENT OF PAKISTAN
CERTIFICATE OF REGISTRATION
Certified that following drug(s) are hereby registered under the Drugs Ordinance/Act, 1976:-
Name of Drug(s).
Name of Manufacturer.
Name of Indenter/Manufacturer's agent/Importer (in case of imported drugs only).
2. This registration shall be valid for a period of five years unless earlier suspended or cancelled.
3. This registration is subject to the conditions specified in the Drugs Ordinance/Act, 1976, and .the
rules thereunder and to the conditions specified in the enclosure.
Date of Registration Secretary
Registration Board
(Seal) Chairman. Registration Board](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-73-320.jpg)
![FORM 7
[See rule 30(6)]
STATEMENT SHOWING QUARTERLY PRODUCTION TO BE SUBMITTED IN DUPLICATE
Name of drug. _________________________
Pharmacological group _________________________
Name of the Firm. _________________________
Address. _________________________
For the quarter ending. _________________________
Pack size. No. of Pack Total quantity in terms of individual units e.g., total No.
of tablets, injections tubes litres etc.
1 2 3
VALUE (in Rs.) Details of Disposal
On trade price On retail
price
Indicate whether supplied through
normal distribution, channels or
exported or supplied to any specific
institution.
Value of raw
materials used
(Active & inactive) (in
Rs.)
4 5 6 7
Total.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-74-320.jpg)


![4.11 Labels
4.12 Batch processing records
SECTION--5
SANITATION AND HYGIENE
5.1 Sanitation
5.2 Hygiene
SCHEDULE B-I
[See rule 16 (6) (b)]
REQUIREMENTS OF PLANT AND EQUIPMENT
(A) The following equipment is required for the manufacture of drugs for external appliances or
suspense:
(1) Mixing tanks where applicable:
(2) Kettles, steam, gas or electrically heated.
(3) A suitable power driven mixer.
(4) Storage tanks or pots.
(5) A calloid mill or a suitable emulsifier or homogeniser, where applicable.
(6) A triple-roller mill or an ointment mill, where applicable.
(7) Liquid filling equipment.
(8) Jar or tube filling equipment, where applicable.
Area of minimum of 200 square feet is required for the basic installation.
(B) The following equipment is required for manufacture of Syrups, Exlixirs and Solutions :--
(1) Mixing and storage tanks.
(2) Mixer.
(3) Filter press or other suitable filtering equipment such as metafilter or sparklet filter or Also-pad
filter.
(4) Water still or Deioniser.
(5) Various liquid measures and weighing scale.
An area of maximum 300 square feet is required for the basic installations.
(C) Equipment for the manufacture of Pills and Compressed Tablets including Hypodermic Tablets.
For efficient operation, the tablet production department shall be divided into the following three
distinct and separate sections situated in different rooms,
(i) Granulating Section;
(ii) Tableting Section;
(iii) Coating Section.
The following equipment is required in each of the three sections :-
1. Granulating Section: (1) Disintegrator, where applicable.
(2) Power Mixer or granulation mixer with stainless steel cabinet
(3} Granular
(4) Oven thermostatically controlled.
2. Tableting Section:
(1) Tablet machine, single punch or rotary.
(2) Pill machine, where applicable.
(3) Punch and dyes storages cabinet.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-77-320.jpg)



![are, in addition, other categories such as drugs miscellaneous pharmaceuticals such as Ferries
Ammonii Citras. Potassium Citras, Glycerin, Paraffin, Oxygen gas, Disinfectant fluids, mechanical
contraceptives, surgical cotton and tinctures which are not listed in this Schedule. The Central
Licensing Board shall, in respect of such categories of drugs, have the discretion to examine the
adequacy or otherwise of factory premises, space, plant, machinery and other requirements having
regard to the nature and extent of the manufacture to carry out necessary modifications in them and,
on the modification. having been made, approve of the manufacture of such categories of drugs. Any
drug so permitted to be manufactured by the Central Licensing. Board shall be deemed to be an
additional category of drug for the purpose of this Schedule.
SCHEDULE B I-A.
[See rule 16 (bb)-7]
CONDITIONS OF FACTORY PREMISES
1. Location and surrounding: The premises should be away from drinking water sources and an
area liable to flooding.
2. (a) Building: Building should be provided with both good general ventilation and protection against
direct sunlight, with easy access for fire-fighting equipment including fire-extinguishers, fire-blankets,
.hose, reels and fire-alarm, etc. Sufficient water must be available for fire-fighting.
(b) Wells: Walls as far as possible should be protected by non-flammable or slow burning material.
(c) Doors; Doors must be fire resistant preferably with self-closing system,
(d) Floors: Floors should be impermeable to liquids, smooth and free from cracks. There should be no
drains at all in plants and in warehouse. If drains are absolutely necessary they must not contract
directly with waterways or public sewers,
(e) Signs: Signs indicating smoking restrictions, location of emergency kits, fire-fighting equipment,
telephone end escape routes must be prominently displayed. Local exhaust system must be
effective,.
3. Personnel: To void intoxication by skin contact, inhalation of fumes, vapours and dust, accidental
ingestion, the protected clothing and equipments, e.g., protective helmet or cloth cap, eye protection
(safety spectacles, goggles or face shield) dust or light fume masks, one piece worksuit with closely
fitting trouser bottoms, rubber or plastic gloves Or gauntlets, rubber or plastic apron, and workboots
with protective toecaps, must be provided.
Staff must not be allowed to go home wearing the same clothing they wore at work; emergency
showers and eye washing facilities must be provided in the premises. Safety instructions should be
strategically displayed in local language. All emergency and safety equipment must be frequently and
regularly checked and maintained to ensure its conditions satisfactory.
4. Medical Services: There must be pre-employment medical; , examination for all staff members
whether working permanently or on contract basis. When organophosphates or carbamates are
handled, pre-exposure baseline blood cholinesterase level must be determined for all operational
staff. Staff regularly engaged in formulation and packing procedures and maintenances must have
their cholinesterase levels checked regularly and detailed records must be kept. The checks should
be carried .out by a properly equipped hospital or laboratory under qualified expert.
"Levels of cholinesterase activity should be interpreted by a doctor, but the following guide might be
helpful :--
(i) A decease of more than 20% in blood cholinesterase activity,. from the pre-exposure value
indicates that the cause should be investigated.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-81-320.jpg)








![SCHEDULE B-III
[See rule 20 (b)]
PARTICULARS TO BE SHOWN IN MANUFACTURING RECORDS
A. Substances Parenteral preparation in general:
1. Serial Number.
2. Name of the drug.
3, Batch Size,
4. Batch number.
5. Date of commencement of manufacture and date when manufecture was completed,
6. Name of all ingredients, quantities required for the batch size, quantities actually used. (All
weighings and measurements shall be checked initiated b¥ the competent person in the section).
7. Control reference numbers in respect of raw materials used in formulation.
8. Date of mixing in case of dry products, e.g., powder, powder mixture for capsule products, etc.
9. Date of granulation wherever applicable.
10. Weight of granules.
11. Date of compression in case of tablets/date of filling in case of capsules.
12. Dates of coating wherever applicable.
13. Records of test to be carried out in case of tablets as under
(a) Average weight every thirty minutes.
(b) Disintegration time as often as practicable.
14. Records of readings taken to check weight variation in case of capsules,
15. Reference to Analytical Report number stating whether of standard quality or otherwise.
16, Records on the disposal of rejected batches and batches with-drawn from the market.
17, Actual production and packing particulars indicating the size and quantity of finished packings,
18. Date of release of finished packings for distribution or sale,
19. in case of Hypodermic tablets and ophthalmic preparations which are required to be manufactured
under aseptic conditions, records shall be maintained indicating the precautions taken during the
process of manufacture to ensure that aseptic conditions are maintained,
20. Signature of the expert staff responsible for the manufacture,
B. Parenteral preparation:
1. Serial Number,
2. Name of the drug,
3. Batch Size,
4. Batch number (if bulk lot is divided into various batches and processed separately, a batch number
distinctly different from that of the bulk lot should be assigned to each of the processed batch),](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-90-320.jpg)



![SCHEDULE C
[See rule 16(c) (iii) and (e)]
1. Sera.
2. Solution of serum proteins intended for injunction.
3. Vaccines.
4. Toxins.
5. Antigen.
6. Antitoxins.
7. Insulin.
8. Pituitary (Posterior Lobe) Extract.
9. Sterilized surgical lignature and sterilized surgical suture.
10. Bacteriophages.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-94-320.jpg)
![SCHEDULE D
[See rule 17(1)]
DRUGS FOR REPACKING
1. Alniminium Hydroxide Gel Dried.
2. Ammonium Bicarbonate.
3. Ammonium Chloride.
4. Ammonium Carbonate.
5. Benzoic Acid.
6. Bismuth Carbonate.
7. Bismuth Subnitrate.
8. Boric Acid.
9. Borax.
10. Caffein and its Salts.
11. Calamine.
12. Calcium Carbonate.
13. Calcium Lactate.
14. Calcium Gluconate.
15. Calcium Hydroxide.
16. Castor Oil.
17. Cetrimide Powder.
18. Chloral Hydrate.
19. Ephedrine Hadrochloride.
20. Ephedrine Sulphate.
21. Ferrous Sulphate.
22. Ferric Ammonium Citrate.
23. Gentian Violet.
24. Glycerin.
25. Iodine.
26. Ichthammol.
27. Kaolin.
28. Liquid Paraffin Heavy.
29. Magnesium Carbonate.
30. Magnesium Hydroxide.
31. Magnesium Sulphate.
32. Methylene Blue.
33. Magnesium Trisilicate.
34. Methyl Salicylate.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-95-320.jpg)
![35. Phenothlazine (B. VET. C.).
36. Pix Carb.
37. Potassium Acetate.
38. Potassium Bromide.
39. Potassium Bicarb.
40. Potassium Chloride.
41. Potassium Citrate.
42. Potassium Iodine.
43. Potassium Permanganate.
44. Procaine Hydro-Chloride.
45. Pulv Gentian.
46. Resorcin.
47. Salicylic Acid.
48. Sentonin.
49. Sena.
50. Sodium Benzoate.
51. Sodium Bicarbonate.
52. Sodium Chloride.
53. Sodium Bromide.
54. Sodium Carbonate.
55. Sodium Citrate.
56. Sodium Iodide.
57. Sodium Metabisuphite.
58. Sodium Potassium Tartrate.
59. Sodium Salicylate.
60. Sodium Sulphate.
61. Sodium Thiosulphate.
62. Soft yellow Paraffin.
63. Sulphonilamide Powder (B. VET. C).
64. Sulphur Precipitated.
65. Sulphur Sublime.
66. Tannic Acid.
67. Zinc Oxide.
68. Zinc Sulphate.
SCHEDULE D-I
[See rule (31)1]
Household remedies including--
Analgesics:
Aspirin and Paracetamol in tablets and liquid forms.
(2) Analgesic Balms/Plasters.
(3) Antiseptics and disinfectants for household use, excluding those containing hormone and
antiniotics.
(4) Antidandruff preparations.
(5) Dental preparations.
(6) Antacid and carminatives:
Compound Effervescent Salts, [--] , Milk of Magnesia.
(7)
(8) Contraceptives.
(9) Miscellaneous.
Fish Liver Oil and its equivalents.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-96-320.jpg)
![SCHEDULE E
[See rule 31 (10)]
DISEASES, ADVERTISEMENT FOR TREATMENT OF
WHICH IS PROHIBITED
1. [Omitted vide S.R.O. 871(I)/78, dated 8th July, 1978.]
2. [Omitted vide S.R.O. 871(I)/78, dated 8th July, 1978.]
3. Venereal diseases.
4. Sexual importance.
5. Amenorrhoea metrorrhagia, memorthagia, metrosalpingitis, ovaritis, fibromas, cysts.
6. Bright’s disease, cataract, glaucoma, epilepsy, [...] lacomotive ataxia, multiple sclerosis, lupus,
paralysis, blindness.
7. Complaints requiring surgical operation (e.g., appendicitis, stomach ulcers, prostatic disorders,
hernias, sinusitis, mastodities.
8. Serious illness liable to endanger the life of the patient (e.g., pneumonai, pleurisy, abscess of the
lungs).
9. Gripe Waters.
10. Cough Preparations.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-97-320.jpg)
![SCHEDULE F
[See rule 5 (2)]
1. DRUG MANUFACTURING LICENCE FEE
(a) For the grant of licence:
Type of licence Fee
By way of basic Rs. 10,000
By way of semi-basic Rs. 10,000
By way of formulation Rs. 25,000
By way of repacking Rs. 15,000
(b) For the renewal of licence
(i) If the application for renewal if made before the expiry of period of validity of licence.
Type of licence Fee
By way of basic Rs. 5000
By way of semi-basic Rs. 5,000
By way of formulation Rs. 12,500
By way of repacking Rs. 7,500
(ii) If the application for renewal is made after the expiry of the period of validity of licence but within
sixty days after expiry of the period validity:
Type of licence Fee
By way of basic Rs. 10,000](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-98-320.jpg)
![By way of semi-basic Rs. 10,000
By way of formulation Rs. 25,000
By way of repacking Rs. 15,000
II. DRUG REGISTRATION FEE
[See rule 26 (3)]
(A) For the grant of Registration Rs. 5,000
(B) For the renewal of Registration
(i) if the application for renewal is made before the expiry of the validity of a
certificate
Rs. 2,500
(ii) if the application for renewal is made within thirty days after the expiry of
the period of validity of a certificate Rs. 5,000
III. FEE FOR ADVERTISEMENT
[See rule 31 (1A) and (1B)]
Application fee for Advertisement. Rs. 1,000 per advertisement](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-99-320.jpg)
![SCHEDULE G
[See rule 30 (11)]
ETHICAL CRITERIA FOR MEDICINAL DRUG PROMOTION
1. Promotion of drugs.- (1) For the purposes of this Schedule, "promotion" means all informational and
persuasive activities by manufacturer and distributors, the effect of which is to induce the prescription,
supply, purchase and/or use of medicinal drugs.
(2) All claims concerning a drug for the purposes of promotion shall be reliable, accurate, truthful;
informative, balanced, up to date, capable of substantiation and in good taste. Such claims shall not
contain misleading, unverifiable statements, omissions likely to induce medically unjustifiable use of a
drug or to give rise to under risks. The word "safe" shall not be used with respect to promotion unless
properly qualified. Comparison of products shall be factual, fair and capable of substantiation.
Promotional material shall not be designed so as to disguise its real nature.
(3) Scientific data in the public domain shall be made available, on request, to prescribers and any
other person entitled to receive it as appropriate to their requirements. Promotion in the form of
financial or material benefits shall not be offered to or sought by health care practitioners to influence
them in the prescription of drugs.
2. Advertisements in any form made to physicians and health-related professionals.-(1) The wording
and illustrations in advertisements to physicians and related health professionals shall be fully
consistent with the approved scientific data sheet for the drug concerned or other source of
information with similar content. The text shall be fully legible.
(2). While introducing the drug to the physician for the first time in shall contain full product
information, on the basis of the approved scientific data sheet or similar document and shall contain,
among others, the following information:-
(a) The generic name(s) of the active ingredient(s);
(b) the content of active ingredient(s) per dosage form or regimen;
(c) the generic name(s) of other ingredient(s) known to cause problem(s)
(d) the approved therapeutic uses;
(e) dosage form or regimen;
(f) side-effects and major adverse drug reactions;
(g) precautions, contra-indications and warnings;
(h) major interactions;
(i) the name and address of manufacturer or distributor; [--]
(j) reference to appropriate scientific literature ; and
(k) Price of the drug, ; and](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-100-320.jpg)

![shall be clearly communicated in advance. The invitation letter should accurately reflect the
presentations and discussions to be held. Entertainment or other hospitality, offered to members of
the medical and allied professions shall be secondary to the main purpose of the meeting and shall be
kept to a modest level.
8. Post-marketing scientific studies, surveilance and disseminaion of information.- (1) The Registration
Board shall be made aware of any post-marketing clinical trials for drugs that are conducted and the
results thereafter as soon as possible.
(2) Post-marketing scientific studies and surveillance shall not be misused as a disguised form of
promotion.
(3) Substantiated information on hazards associated with the drug shall be reported to the
Registration Board as a priority.
9. Packaging and labelling.- Appropriate information being important to ensure the rational use of
drugs, all packaging and labelling material shall provide information consistent with that approved by
the Registration Board and if no such approval is available it shall be, consistent with that approved by
the drug regulatory authority of the country from which the drug is imported or other reliable sources
of information with similar content. Any wording and illustration on the package and label shall
conform to the principles of ethical criteria enunciated in this Schedule.
10. Information for patients contained in package inserts, leaflets and booklets.- (1) Adequate
information on the use of drugs shall be made available to the patients where it is necessary for
rational use of a drug. In package inserts or leaflets the manufacturers or distributors shall ensure that
the information reflected is correct. If package inserts or leaflets are used for promotional purposes,
they shall comply with the ethical criteria enunciated in this Schedule. The wording of the pcakge
inserts or leaflets, if prepared specially for patients, shall be in lay language subject to the condition
that the medical and scientific content is properly reflected.
(2) In addition to approved package inserts and leaflets wherever available the preparation and
distribution of booklets and other information material for patients and consumer shall also comply
with the ethical criteria enunciated in this schedule.
[No. F. 8-1/90--AU (Vol-11.)]
DR. F.R.Y. FAZLI,
Deputy Director General (Pharmacy)/Drugs Controller.
(S.R.O. 1362(I)/96 28.11.1997)](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-102-320.jpg)

![Page | 1
DRUGS
(APPELLATE BOARD) RULES,
1976
S. R. O. 595 (1)/76, dated 2lst June, 1976: In exercise of the powers conferred by
Section 43 of the Drugs Act, 1976 (XXXI of 1976), the Federal Government is pleased to
make the following rules, the same having been previously published as required by sub-
section (3) of the said section, namely :--
1. Short title and commencements:
(1) These rules may be called the Drugs (Appellate Board) Rules, 1976.
(2) They shall come into force at once.
1
[1A. Conflict of interest. - A member of the Appellate Board or his representative shall
not participate in the proceedings or express any opinion in cases in which conflict of
interest arises in respect of matters, dealt with by such member or representative,
specified in clause (a) of sub-section (5) of section 11 of the Drugs Act , 1976 (XXXI of
1976).]
2. The Appellate Board: 2
{(1) The Appellate Board shall consist of the following
members, namely:--
3
[(a) Chief Executive Officer of the Drug Regulatory Authority of Pakistan,
who shall be its ex-officio Chairman;]
(b) Director Legal Affairs, Drug Regulatory Authority of Pakistan, who shall
be its ex-officio Secretary;
(c) Secretaries, Departments of Health of the Governments of Punjab, Sindh,
Khyber Pakhtunkhwa, Balochistan and Gilgit Baltistan or their nominees not
below the rank of an officer in BPS-20, who are experts in medicine,
pharmacology or pharmacy and a representative from Federally Administered
Tribal Areas, who shall be ex-officio members;
(d) one professor of medicine or surgery, to be nominated by the Authority;
(e) one expert in pharmaceutical manufacturing, to be nominated by the
Authority;
(f) one professor of pharmacology, to be nominated by the Authority;
(g) one professor of pharmacy, to be nominated by the Authority; and
(h) a co-opted expert in the field related to a specialty case before the
Appellate Board, to be nominated by the Chairman of the Appellate Board.}
(2) The members, other than ex-officio members, of the Appellate Board shall hold
office for a period of three years and shall be eligible for renominations.
_____________________________________________
1. Inserted by S.R.O.664(I)/2005, dated 25-06-2005.
2. Substituted by S.R.O. 475(I)/2013, dated 29-05-2013.
3. Substituted by S.R.O. 674(I)/2015, dated 10-07-2015.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-104-320.jpg)
![Page | 2
(3) The Appellate Board shall meet as and when required to perform its functions.
(4) The Appellate Board shall have powers to appoint a Committee of Experts for
detailed investigation of any matter and report to the Board.
(5) No act or proceeding of the Appellate Board shall be invalid merely on the ground of
the existence of any vacancy in, or any defect in the constitution of the Board.
3. Powers of the Appellate Board: The 4
[ex-officio] members of the Appellate Board
shall exercise all the powers of an Inspector without restriction as to area, and such other
powers as may be necessary to perform their functions.
4. Procedure of Appeal: (1) Any person aggrieved by a decision of the Registration
Board, the Central Licensing Board 5
[, Pricing Committee] or a licensing authority may,
within sixty days of receipt of such decision, submit an appeal to the Appellate Board.
(2) An application for appeal under sub-rule (1) shall be 6
[in triplicate and be]
accompanied by a copy 7
[each of the application which has been rejected] and the
decision appealed against, and shall contain all material statements and arguments relied
on by the appellant and a fee of 8
[fifty] thousand rupees for each application.
9
[(2a) In case of appeal against the decision of the Registration rejecting an application
for registration of drug or for fixation of price the application shall be accompanied by a
statement in form A or form B of the Schedule to these rules, as the case may be.]
(3) The Appellate Board shall transmit a copy of the application for appeal referred to in
sub-rule (2) to the Registration Board or the Central Licensing Board 10
[, Pricing
Committee] or the licensing authority against whose decision the appeal has been made
and such Board or authority shall, on demand, produce before the Appellate Board the
record of the case leading to the decision.
(4) The Appellate Board shall, after giving the appellant an opportunity of being heard,
pass such orders as it thinks fit and such orders shall be final.
5. Revision: The Appellate Board may, of its own motion at any time, call for the record
of any case for the purpose of satisfying itself as to the correctness, legality or propriety
of such order and may pass such order in relation thereto as it thinks fit.
________________________________________________
4. Inserted by S.R.O. 475(I)/2013, dated 29-05-2013.
5. Substituted by S.R.O. 475(I)/2013, dated 29-05-2013.
6. Words inserted by S.R.O. 12(I)/77, dated 07-06-1978.
7. Substituted by S.R.O. 1453 (I)/78, dated 16-12-1978.
8. Substituted by S.R.O. 463 (I)/2013, dated 29-05-2013.
9. Sub-rule 2(a) inserted by S.R.O 1453(I)/78, dated 16-12-1978.
10. Inserted by S.R.O. 475(I)/2013, dated 29-05-2013.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-105-320.jpg)
![I
2
4
5
Name ofapplicant
Name and exact composition ofthe drug.
Name of the manufacturer-
Reasons for rejection ofthe application as communicated by the
Registration Board and arguments in support ofthe appeal.
Comparative statement ofprices ofother competitive and
pharmacologically equivalent registered products ftom different
source in support ofthe economic value ofthe drug:-
Name of
Product
Name of
manufacturer
Price per unit Estimated cost of
full treatment
6. Summary ofcompetitive study and advantages ofthe safety and efficacy
with other therapeutically equivalent products.
7. In case ofa drug proposed to be manufactued locally, the capacity ofthe
manufacturing section:
lnstalled capacity Utilized capacity Un-utilized capacity
8. Pharmaceutical dosage forms ofdrugs and total number ofdrugs in each
such form in respect ofwhich registration has been granted to the
manufacturer: -
Dosage form Total number ofdrug
Tablets
Ampoules
Vial
Syrup
9. List ofdrug manufactwed in each section elc. in respect ofwhich
registration has been granted along with their registration number, stsength
and pickings.
10. Has at any time any product ofthe manufacturer been declared
substandard. If so please give details ofthe product and ffi thereof.
FORM"A'
[Under sub-rule( 2a) of Rule 4 ofthe Drugs (Appellate Boa l) Rules, 1976]](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-106-320.jpg)
![I Has the manufacturer ever been convicted ofviolating any ofthe
provisions ofthe Drug Act 1976, or the rules made thereunder?
12. Have stability studies and, where applicable bioavailability studies, been
conducted? Ifso, please enclose copies ofreport.
13. Name, qualifications and designations oftechnical staffresponsible for
manufacturing and quality conEol.
t4. Has the manufactwer satisfactory facilities for quality control and internal
and extemal specifications for the drug in respect of which appeal is
preferred? lfso, please give details.
15. Is it a new drug? If so, enclose reports of clinical trial and other material as
required by rule 29 ofthe Drugs (Licensing, Registering and Advertising)
Rule 1976.
16. In case ofan imported drug, does the appellant posses the following
documents fiom the competent authorities specified at serial No. 20 and 2 t
of Form 5-A Application form for registration of drugs in Schedule A to
the Drugs (Licensing, Registering and Advertising) Rule 1976, namely:-
a) Certificate of fee sale and G.M.p. and
b) Certificate ofregistration with a Photostat copy regarding
conditions ofregistsation and labeling in the country oforigin.
INSTRUCTION TOR FILLING THE FORM
l. The appeal form should be in foolscap.
2. Three copies ofthe form should be submitted
3. In the statement ofcomparative study ofprices and the slatement of
comparative study of safety and efficacy, the information in respect of
the drug under appeal should be given first and about other products
should follow in a tabulated form serial-wise.
4. Serial No. 7 is appticable only in respect ofa locally manufachred
drug and not to a drug to be imported in frnished form.
5. Capacity means the capacity ofnormal working ofa manufacturing
section on single-shift basis, and is to be given in terms oftotal
number ofunits (i.e. tablets, ampoules, vials, bottles with specified
sizes) in each section.
6. For the purpose ofSerial No.8, the number ofdrugs will be counted
on the basis ofnumber ofregistrations granted by the Regisrration
Board in case ofregistered drugs, or the Dumber of items on the basis
ofthe various dosage forms, as the case may be.
I inse edvi& Notificotion S.RO. 1453U)n8, dated I6t Decenbe l97E]](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-107-320.jpg)
![FORM'8"
[Under sfi-rule( 2a) of Rule 4 of the Drugs (Appellate Board) Rules, 1976J
l. Name ofthe product
Packing
2. Costing statement:
(l) Cost ofraw material (give details ofindividual components).
@ Cost ofpacking material ((give details of individual components).
(3) Direct labour.
(4) Over-head charges-
(a) Factory over heads.
(b) Sales Promotion.
(c) Miscellaneous.
(with break up showing minor items).
(5) Profit.
(6) Ex-factory price.
(7) Trade price.
(8) Maximum retail price.
3. Comparative statement of prices of competitive and pharmacologically
equivalent products registered from other sources.
I intefledli& Notifica,ion S.RO. 1453(W8, dare.l td Dece ber, 1978]
Cost item Rate (lnvoice
enclosed)
Name and
address of
the exporter
Ingredients of preparation
Quantity Actual cost
as per
column
I 2 3 4 5
Name of the
Product
Price per unit Estimated cost of full
treatment in case of
other
phannacologically
equivalent Droduct
I 2 J 4
Source](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-108-320.jpg)


![(a) to inspect not less than twice a year, all premises licensed for the
manufacture of drugs including the plant and the process of manufacture, the
means employed for standardising and testing the drugs,, the methods and places
of storage, the location, construction and administration of the establishment
likely to affect the potency for purity of the product, records and registers and to
satisfy himself that the conditions of the licence and the provisions of the Act and
the rules made thereunder, are being observed ;
(b) to inspect from time to time establishment licensed for the import, export or
sale of drugs and to satisfy himself that the conditions of the licence are being
observed;
(c) to send forthwith to the licensing authority after each inspection a detailed
report indicating the conditions of the licence and provisions of the Act and the
rules made thereunder which are being observed and the conditions and
provisions, if any, which are not being observed;
(d) to take samples of any drug which he has reason to suspect that it is being
manufactured, stocked, sold or exhibited for sale in contravention of the
provisions of the Act or the rules made thereunder. and send them for test or
analysis;
(e) to investigate any complaint in writing which may be made to him; [ ...... ]
(f) to institute, if necessary, prosecutions in respect of breaches of the Act and the
rules made thereunder. and
(g) to give advice to pharmaceutical industry on technical matters pertaining to
the manufacture of drugs in accordance with good manufacturing practices with
a view to improve the standard of industry and quality control of drugs;](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-111-320.jpg)










![(q) “seller” means the seller of a drug; and
(p) “wholesale” means sale to a person, buying for the purpose of
selling again who is the authorized agent of a manufacturer or
importer or indenter.
(2) A word or an expression used in these rules but not defined shall
mean the same as defined in the Act.
CHAPTER II
PROVINCIAL BOARD, DISTRICT BOARD,
GOVERNMENT ANALYST AND INSPECTOR
3. Provincial Quality Control Board.– (1) The Board shall consist of the
following:
(a) Secretary to the Government, Health Department, ex
officio member and chairperson;
(b) Additional Secretary (Technical) to the Government, Health
Department, ex officio member and vice-chairperson who
shall act as chairperson in the absence of the Secretary
Health;
(c) Provincial Drugs Controller of the Government or a senior
most officer of the Provincial Drugs Control administration
who shall be a pharmacy graduate, Health Department, ex-
officio member;
(d) a pharmacy professional who holds a graduate or higher
degree in Pharmacy and has more than five years
professional experience, appointed as a private member
by the Government for a term of four years;
(e) a pharmacologist preferably a professor of pharmacology,
appointed as a private member by the Government for a
term of four years;
(f) a professor of medicine, appointed as a private member by
the Government for a term of four years;
(g) District Coordination Officer of a district, ex officio member,
in respect of cases pertaining to the district;
(h) Executive District Officer (Health) of a district, ex-officio
member, in respect of cases pertaining to the district; and
(i) a pharmacist of the Government, Health Department, in a
district, appointed [ ]by the Government for a term of four
years who shall be the Secretary of the District Board.
(2) The Government shall appoint a secretary of the Provincial
Board, who holds a graduate or higher degree in pharmacy and has at least ten
years professional experience who shall also be member of the Provincial
Board.
(3) The Government may appoint a pharmaceutical expert and an
expert of medicine as members of the Provincial Board in respect of a district
for a term of four years.
2](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-122-320.jpg)








![SCHEDULE A
[See rule 2(n)]
FORM 1
[See rule 5(1)]
MONTHLY REPORT FROM INSPECTOR
For the month of __________
(A) SUMMARY OF INSPECTIONS
Place
Inspected
No of Firms
Inspected
No of Firms found
violating law
------Specify main
offences
No of
samples
drawn, if
any
Remarks
Manufacturer
s
Pharmacies &
medical
stores
Others,
please specify
(B) DETAILS OF VIOLATIONS IN RESPECT OF DRUGS
Report of samples of drugs not in compliance with law
Name
of
Drug
Regd No and
Manufacturer’s
Name
Batch
No
Place of
taking
sample
Date of
dispatch
& Name
of Lab
Date of
receipt
of test
report
with
nature
of result
Action
taken
including
details of
seizure
and sale
restriction
(C) Copy of inspection report of Pharmaceuticals Manufacturing units should be
supplied alongwith comments about the compliance of GMP.
FORM 2
[See rule 5(1)]
DRUGS TESTING LABORATORY -----------
PROGRESS REPORT FOR THE MONTH OF --------
No of
samples
in the
beginning
of the
month
Samples
received
during
the
month
Total Tested Samples
up to
standard
with
percentage
Samples
below
standard
Details
of
samples
pending
for more
than 60
days
Rema
rks /
Reas
on
New Ol
d
Total
Spurious =
Substandard =
Adulterated =
Drugs /Medicines of other
systems found to contain
allopathic ingredients =
11](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-131-320.jpg)

![FORM 3
[See rule 9]
Order under section 18(1) of the Drugs Act, 1976 regarding person not to
dispose of stock in his possession.
Whereas I have reason to believe that the stock of drugs, article or other
things in your possession detailed below contravenes the provisions of section
…………. of the Drugs Act, 1976. Now, therefore I hereby direct you not to
dispose of the stock for a period of ………….. days from this date.
Date………….. Inspector……………………
Details of stock of drugs.
Date…………………. Inspector…………………….
FORM 4
[See rule 10(1)]
Intimation of purpose to person from whom the sample (s) is taken.
To
………………………….
I have this day taken from the premises of ……………………..situated at
samples of the drugs specified below for the purpose of test/analysis.
Date………………………… Inspector
…………………..
Details of samples drawn
Name of
drug
Name of
manufacturer
Registration
No
Batch
No
Quantity Bill No Mfg & Exp date
Date………………………… Inspector
…………………..
FORM 5
[See rule 10(1)]
Receipt for stock of drug and other material articles seized under section
18(1) of the Drugs Act, 1976.
The stock of drugs materials/articles detailed below has this day been
seized by me under the provisions of clause (f) of sub-section (1) of section 18
of the Drugs Act, 1976 from the premises of…………………………………….
Situated at ……………..
Date………………………… Inspector
…………………..
Details of Drugs, other material and articles seized including;
Sr No Name of
drug
Batch
No
Name of
manufacturer
Quantity Reason for
seizure
13](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-133-320.jpg)

![FORM 6
MEMORANDUM TO GOVERNMENT ANALYST)
[See rule 10(2)]
Serial No of Memorandum ……………………………………………….
From
-----------------------
-----------------------
To
The Government Analyst
--------------------------------
The portion of sample/container described below is sent herewith for test
or analysis under the provisions of clause (i) of sub-section (3) of Section 19 of
the Drugs Act, 1976.
The portion of sample/container has been marked by me with the
following marks.
(Seal---------)
Details of portion of sample/container with name of drug which it
purports to contain including;
Name of
drug
Name of
manufacturer
Registration
No
Batch
No
Mfg &
Exp date
Quantity
Dated ………………… Inspector……………….
=================================================
FORM 7
DRUGS ACT, 1976 AND DRUGS RULES, 1988 FRAMED THERE UNDER
[See rule (11)(1)]
Report of Test/Analysis by Government Analyst, Punjab
1. Name of Inspector of Drugs from whom received
…………………
2. Serial Number and date of Inspectors memorandum
……………….
3. Date of
receipt……………………………………………………….
4. Name of Drug purporting to be contained in the sample
……………
5. The condition of the
seals……………………………………………
6. Result of test/analysis with specifications
applied………………….
In the opinion of the undersigned, the sample referred to above is of
standard quality as defined in the Drugs Act, 1976 and rules there-under;
Is adulterated/substandard/misbranded/spurious, as defined in the
Drugs Act, 1976 for the reason given above.
(Please score out which is not applicable)
15](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-135-320.jpg)

![FORM NO. 8(A)
{See rule 15 (1)}
Application for the license to sell, store and exhibit for sale & distribute
drugs by way of pharmacy.
1. I / We
____________________________________________________.of M/S
_______________________________________hereby apply for
Licence of Pharmacy;
2. The sale of drugs will be under the personal supervision of;
(name, registration No, NIC No & address with qualification).
1.________________________________________________________
________________________________________________________
2.________________________________________________________
________________________________________________________
3. I / We am / are submitting herewith the following documents;
A) Testimonials of the person (s), registered under section 24(1)(a) of the
Pharmacy Act 1967, who has agreed to personally supervise the sale of
drugs for licence in Form 9 (pharmacy)and the proprietor (s)
i) three attested copies of registration certificate issued by a
pharmacy council.
ii) four attested copies of National Identity Card & passport size
photographs of the proprietor (s) and person (s) incharge who has
agreed to personally supervise the sale of the drugs.
iii) Affidavit of the person who will supervise the sale of drugs and
the proprietor, duly verified, to the effect that they:-
a) shall comply with the provision of the Drugs Act, 1976
and rules framed there under;
b) have not been convicted of any offence from any Court of
law. [See rule 19 (1) (e)];
c) shall inform the Licensing Authority for any change in
supervisory staff etc.
d) are not working in any government / semi government /
autonomous organization.
e) shall not sell / stock any expired, spurious, substandard,
unregistered misbranded, counterfeit or any drugs in violation
to the drugs laws in force.
B) Plan indicating the exact location and specification of the
premises including covered area, dimensions, signboard, air
conditioning and refrigeration facilities and addresses of go-down
(if any).
C) Treasury receipt / challan No & dated ------- amounting to
Rs.-------
in the Head of Account 1252-Health & Other receipts.
Dated:___________
17](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-137-320.jpg)

![FORM NO. 8(B)
{See rule 15 (1)}
Application for the license to sell, store, exhibit for sale & to distribute
drugs excluding the drugs specified in Schedule “G” by way of Medical
Store
1. I / We
____________________________________________________.of M/S
_______________________________________hereby apply for
Licence of Medical Store;
2. The sale of drugs will be under the personal supervision of;
(name, registration No, NIC No & address with qualification).
1.________________________________________________________
________________________________________________________
2.________________________________________________________
________________________________________________________
3. I / We am / are submitting herewith the following documents;
A) Testimonials of the person (s), registered under section 24(1)(a) or (b) of
the Pharmacy Act 1967, who will supervise the sale of drugs for
licence in Form 10 (medical store)and the proprietor (s); and
Testimonials of the person (s), registered under section 24(1) of the
Pharmacy Act 1967, who will personally supervise the sale of drugs for
licence in Form 10 (medical store)and the proprietor (s).
i) three attested copies of registration certificate issued by a
pharmacy council.
ii) four attested copies of National Identity Card & passport size
photographs of the proprietor (s) and person (s) incharge who has
agreed to personally supervise the sale of the drugs.
iii) Affidavit of the person who will supervise the sale of drugs and
the proprietor, duly verified, to the effect that they:-
f) shall comply with the provision of the Drugs Act, 1976
and rules framed there under;
g) have not been convicted of any offence from any Court of
law. [See rule 19 (1) (e)];
h) shall inform the Licensing Authority for any change in
supervisory staff etc.
i) are not working in any government / semi government /
autonomous organization.
j) shall not sell / stock any expired, spurious, substandard,
unregistered misbranded, counterfeit or any drugs in violation
to the drugs laws in force.
B) Plan indicating the exact location and specification of the
premises including covered area, dimensions, signboard, air
conditioning and refrigeration facilities and addresses of go-
down (if any).
C) Treasury receipt /challan No & dated ------- amounting to Rs.-------
in the Head of Account 1252-Health & Other receipts.
Dated:___________
19](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-139-320.jpg)





![SCHEDULE ‘B’
[See rules 20]
NARCOTICS PSYCHOTROPIC, ANTI DEPRESSANT AND OTHER
CONTROLLED DRUGS
Acetorphine Acetylmethadol Allyiprodine
Alphacelylemethadol Alphamethadol Alphaprodine
Atileridine Benzethidin Benzylmorpine
Betacoylethadol Betaprodine Betamethadol
Betaprodine Bezitramide Bezodiazepine
Buprenorphene
Cannabis Clonitazone Coca Leaf
Codoxime Concentrate of poppy
straw
Desmorphine
Dextromoramide Diampromid Diethylthiambutene
Difenoxin Dihydromorphine Dimenoxadol
Dimepheptenol Dimethylthiambutene Dioxaphetyl butyrate
Diphenoxylate Dipipanone Dextropropxyphene
Dorotebano Ecoonino Ethylmothylhiambutone
Etonitazene Etorphine Etoxeridne
Fantayl Furethidine Heroin
Hydrocodone Hydromorphinof Hydromorphone
Hydroxyperthidine Isomethadone Katobemidone
Levomethorphen Levomeramide Levophenacylmorphen
Levorphanol Methazocine Methadone
Methadone intermediate Methyldeserphine Methyldihydromorphine
Metopen Moramide intermediate Morpheridine
Morphine, Morphine Methorbromide and other pentavalent nitrogen morphine
derivatives include in particular the morphine-N-oxide derivatives, one of which
is Codeine-N-oxideand the Drugs listed in schedule to CNS Act 1997.
Morphine M - oxide. Myrophine Nicomorphine
Noracynethadol Norlevorphanol Normathadone
Normorphine Norpipnene Opium
Oxycodone Oxymorphone Pethidine
Pethidine intermediate
A
Pethidine Intermediate B Pethidine Intermediate
C
Phenadoxone Phenampromide Phenazocine
Phenomorphan Phenoperidine Piminodine
Piritrameide Propheptazine Properidine
Pentazocine Recamethorphane Recomoramide
Racemorphan Surfatnil Steroids except topical
preparations
Thebacon Thebaine Tramadol
Trimeperidine Acetyl di hydro codein Ethlmorphine
Nicocodiene Norcodein Pholcodein
Propyiyam
International Non
Proprietary
Names
Other non
proprietary or
trivial names
Chemical Names
DET
DMHP
N N Diethyl trptamine, 3-(1,2
dimethyl heptyl) 1 hydroxy 7,8,9,10
25](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-145-320.jpg)
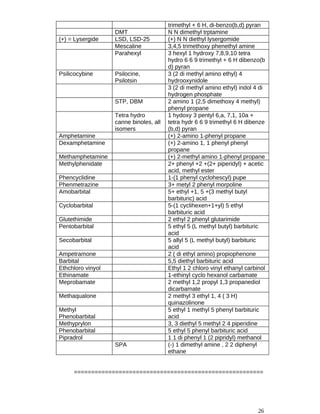
![SCHEDULE ‘C’
[See rules 13 (2)]
1 Short conclusion/judgment (without experimentation) Rs.
50
2 Preliminary examination of character e.g. color taste smell
form solubility, miscibility, etc.
115
3 Clarity of solution,
(1) Physical Examination 70
(2) Chemical Examination 115
4 Completeness of solution 150
5 Identity test, chemical
(A) (a) Inorganic substance 120
(b) Organic substances 125
(B) Unknown sample
(a) Inorganic 170
(b) Organic 225
(i) Element each 150
(ii) group each 130
6 Leakage test Injectable 140
7 Disintegration test, dissolution test, weight variation
(uniformity of weight) uniformity of diameter, etc.
340
8 Determination of solubility quantitatively in one solvent 260
9 Determination of melting point
(a) In-capillary 130
(b) In non declared substances 240
10 Micro melting point in non-declared substance 250
11 Crystallizing point, freezing point, setting point and
solidifying point each
200
12 Distillation range and boiling point, etc. 140
13 Determination of water/humidity
(a) In ointments. 140
(b) In other material 230
14 Residue after evaporation or loss on drying Quantitatively 130
15 Weight per ml, density, specific gravity, etc. 240
16 Determination of viscosity 250
17 Determination of jelly strength. 240
18 Determination of ash, acid insoluble ash, water soluble ash
sulphated ash, alcohol soluble extractive total solids, etch
each.
240
19 Readily carbonisable substances test 225
20 Determination of alcohol in the preparations. 160
21 Extraction with organic solvents 290
22 Continuous extraction of drugs 385
23 Isolation by distillation 260
24 Steam distillation 240
25 Vacuum distillation 350
26 Determination of unsaponifiabe matter free menthol, cineol,
total balsamicacids, etc. each
250
27 Determination of Acid value, Iodine value, saponfication
value Acetyl value, eaters value, etc, each
150
27](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-147-320.jpg)
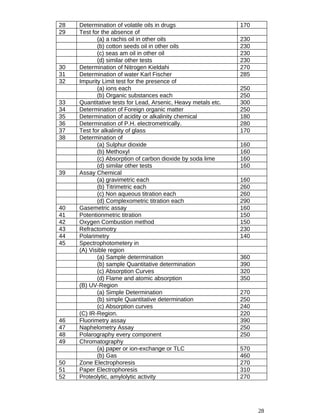
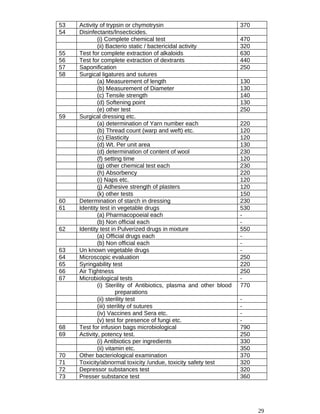
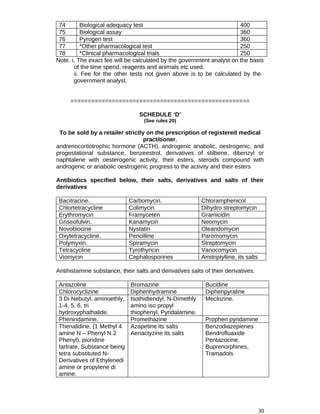
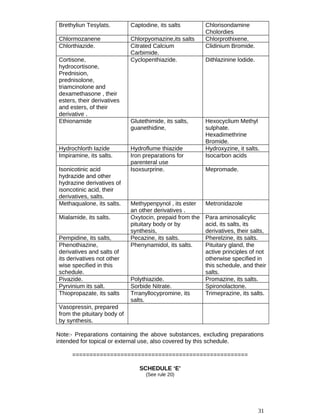




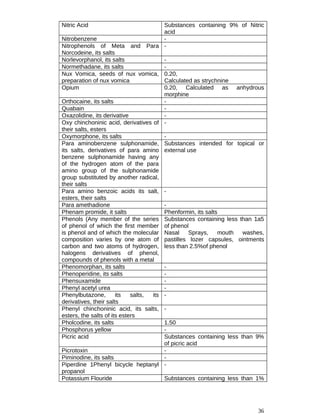
![of Pot fluoride
Potassium Hydroxide -
Procaine, salts of procaine Combination of procaine with
antibiotics
Proheptazine, its salts -
Propoxyphene, its salts -
Recomthorphan, its salts -
Reserpine, its salts, its derivatives,
their salts
-
Salicylconchoninic acid, its salts,
esters, the salts of its esters
-
Savin oil of sodium fluoride Substances containing less than 1%
of sodium fluoride
Sodium Hydroxide Substances containing less than 12%
of NaOH
Sodium Nitrate -
Strophanthus, its Glycosides -
Sulphuric Acid Substances containing less than 9%
of Sulphuric Acid
Thallium, its salts -
Thiocarbonalide -
Thyroid, glands, the active principle
of their salts
-
Tolbutamide -
Tribromomethyl alcohol -
Tri(2 Chlorethyl) Amines, its salts -
Tri ethylene thio phosphoramide -
Trimeperidine, its salts -
Tropine di phenyl methyl esters, their
salts
-
Roxidone -
Nephosphide -
============================================
SCHEDULE ‘F’
[See rule 19(1) (c)]
LIST OF MINIMUM REQUIREMENTS FOR A PHARMACY
1. Entrance; The front of a Pharmacy shall be an inscription
“Pharmacy”.
II. Premises; The premises of a pharmacy shall be separated from room
for private use. The premises shall be built dry, well lit and ventilated and shall
of sufficient dimensions to allow the goods in stock, especially drugs and
poison to be kept in a clearly visible and appropriate manner. The area of the
section to be used at dispensing department shall not be less than 6 sq Meters
for one person working therein with additional 2 sq Meters for each additional
person. The height of the premises shall at least be 2.5 sq Meters.
The floor of the Pharmacy shall be smooth and washable. The walls
shall be plastered or tiled or oil painted so as to maintain smooth durable and
washable surface devoid of holes cracks and cervices.
A Pharmacy shall be provided with good quality of water. The dispensing
department shall be separated by a barrier to prevent the entry of public.
37](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-157-320.jpg)

![All records and register shall be maintained in accordance with the laws
in force
Any container taken from the poison cup board shall be replaced therein
immediately after use and the cupboard locked. The keys of the poison
cupboard shall be kept in the personal custody of the responsible person.
Drugs when supplied shall have labels conforming to the provisions of
laws in force.
Note; The above requirements are subject to modification or the directions of
the Licensing Authority, if the Authority is of the opinion that having regards to
the nature of drugs dispensed, compounded or prepared by the licensee it is
necessary to relax the above requirements in the circumstances of a particular
case.
================================
Schedule G
[See rule 20(1)(e)]
DRUGS NOT TO BE SOLD/STORED BY LICENCEE IN FORM NO.10
1. Antileprosy
i Rifampicin Injection iv Ethionamide
ii Dapsone v Prothionemide
iii Clofamazine
2. immunological products, Vaccines, Sera / Anti Sera
i Anthrax Vaccine ix Rubella Vaccine
ii BCG Vaccine x Pneumococcal vaccine
iii Botulisms Antitoxin xi Poliomyelitis Vaccine
iv Cholera Vaccine xii Smallpox Vaccine
v Diphtheria Vaccine xiii Typhoid Vaccine
vi Influenza Vaccine xiv Immunoglobulins
vii Measles Vaccine xv Rabies Vaccine
viii MMR Vaccine xvi Homophiles Influenza-
Type B Vaccine
3. Products Related with Malignant Diseases and Immunosupression
i Folinic Acid xiii Mitozantrone
ii Doxorubicin HCl xiv Methotrexate
iii Mercaptopurine xv Vinblastine
iv Thioguanine xv Carboplatin
v Vincristine xvii Bleoimycin
vi Cisplastin xviii Dactinomycin
vii Busulphan xix Chlorambucil
viii Carmustine xx Dacarbazine
ix Lomustine xxi Amasascrine
x Cyclophosphamide xxii Azathioprine
xi Melphalan xxiii Cyclosporin etc
xii Fluorouracil
4. Drugs of Anesthesia and Inhalation Anesthetics
i Propofol viii Mitazolam
ii Enfluran ix Naloxone Hcl
iii Isofluran xv Vancuronium
iv Halothane xi Pancuronium
v Bupivacain xii Tubocuraine
vi Thiopentone xiii Suxamethonium
vii Benzodiazepine xiv Neostigmine
39](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-159-320.jpg)



![?4, eh;"-.
1 'l'
PAlLl'-l THE PUNJAB WEEKLY GAZETTE FEBRUARY 10,2OIO 951
GOVERNMENT OF THE PUI"IJAB
HEALTH DEPARTMENT'
Dated 2nd FebruarY 2010
NOTIFICATION:
No. SO (DC) 814/2003 (A-97) (P) ln exercise of powers conferred upon him under section 44. of
il; ild; e"t, reZO (XiXl oi iSZO; *ni"f, empowers the Provincial Government to make rules
under thL Act subject to previous publication, .
And whereas tfre-Governor of the Punjab was pleased to make the Punjab Drugs Rules
1988 and substitute tn", *itn tf'" Punjab Drugs Rules, 2007, under the said provision;
- ..
And whereas tn"'Cor"inoi or tn" piunjab is further .pl."a-s."d
to make the following
amendments in the punjlbiiug. Rrr"., 2OO7
-aier
previorls publication of these arnendments:
" - _
EIVTEruDMENTS IN PUNJAB DRUGS RULES' 2OO7
ln Rule 1-
i; ;c 1, sub rule (3) for the word
,,three,,, the word ,,ten" shall be substituted.
in clause (k) after the word "prescription" the following shall be
i
I
$
:
I
i
I
i
1
added;
,,ar distributed in case of authorized agent of manufacturer, indenter or impoder"
tn rute 2,
"uu
,ure-iri-rii"i
"rrr."-tpl
the folowing new clause (q) shall be inserted;
,,(q) ,,wholesale"'means sale to a person, bulng for the. purpose of selling again
who is tire authorized agent of a manufacturer or importer or indenter'
3. ln Rule 3-
ln rule 3, sub rule (1), clause (b) after the word "vice-chairperson" the following shall be
inserted;
..whoshallactaschairpersoninth-eabsenceofthesecretaryHealth',.
ln rule 3, .rO |.uL tJi clause (c;, after the word "Government", the following shall be
inserted;
.,or a senior most officer of the provincial drugs control administration who shall
be a pharmacY graduate".
lnr:ule3,suorutelt;,clause(i),thewords,.asaprivatemember',,shallbeomittedand
after the word "year", tL *'orJ., ;*ho shall be the Secretary of the district board" shall be
inserted.
, ln rule 3, sub rule (2), after the word
..experience,,, the following shall be inserted:
"who shall also be'member of provincial board"'
ln rule 3, atter suO iute (5), the foilowing nev'' sub rule shall be inserted:
"(6) the Uorra ,i"!'"o opt any olhJr. Oualified expert having formal training and
eiperience in the pharmaceutical field"
4. ln Rule 18-
ln rule 18, the sub rule (1), shall be substituted with the following;
,,A licence i"".]"j;; ';*"*"0 under these rules shall unless suspended. or
cancelled earlier, remain in force for two years from the date of issue" I
5 ln Rule 18- hall be substituted with
lnrulelS,subrule(2)aftertheword..within',,theword..Sixty''S
ln Rule 2-
ln rule 2, sub rule (1),
lhe word "Thirty"
i lrt Rule 19-
ln rule 19 (1) (e) after the word "more" the words "for manufacturing or selling
spurious drugs" shall be inserted'^ rt L^ arrrra^.
tn rutJ"t-g til "it"|.
sub rute (f) the following proviso shall be added;
,f roiiOoi tirai provision ii tt i" rule foi iicences.already issued shall come into
force after terr years from the notification of these rules"
secretary
f n ,"uf" f'S, sub rule (2), the word, "s6cretary of the.provincial board or the
eif ttre district board" shall be substiiuted with t-he word "by a committee comprising of
SecretaryoftheDistrictBoardortheareaDrugslnspector,'.
7. ln Rule 20-
tn ruie zo, sub rule (6), after clause (b), the following proviso shall be added;
,,provided that no pharmacy or medical store shall be allowed except and in
accordance with the provisions of these rules'
8. ln Schedule A-
f n foim b at serial No '1
, after the word "and sell", the word "or distribute" shall be
inserted
9. ln Schedule A-
4 f n noim i0 at seriat No 1, after the word "distribute" the words "or sale by way of
wholesale" shall be inserted'
. ay rHE oRDER oF THE GovERNoR
$ECRETARY TO THE GOVERNMENT OF THE PUNJAB
HEALTH DEPARTMENT](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-163-320.jpg)


















![(2) Each company shall provide a detailed summary of all expenditures incurred on
institutions or healthcare professionals on account of marketing, honoraria, travel, subsistence
expenses, grants and any other related financial transaction to the relevant tax authorities as
well as to the Authority on annual basis as per Schedule. This summary shall include the full
name, National Tax Number and National Identification Card Numbers of all individuals who
have benefited from such support.
(3) Healthcare professionals shall disclose any conflict of interest, funding received
and other relevant activities during the course of interaction, with companies or company
representatives, to their institutions.
15. Contravention and punishment.– Whoever, himself or by any other person on
his behalf contravenes any provision of these rules shall be punishable in accordance with the
provisions of the Act and Schedules made there under.
16. Cognizance of offence.– (1) Cognizance of offence shall be in accordance with
the provisions of the Act and Schedules made there under.
(2) In case of complaints and non-compliance of the rules by a healthcare professional
or the healthcare industry, the recommendation shall be referred to concerned Division of the
Federal Government, department of concerned governments, regulatory bodies or directorate
to take necessary action as per prevailing laws.
(3) All such complaints shall be handled and processed by the Authority through
relevant Divisions.
SCHEDULE
[see rule 14(2)]
DETAILS OF EXPENDITURE
Company Name: Turnover: PKR
Financial Year:
Sr.
Advertising
Physician’s
Sample
Promotional
Printed
Material
Give
Aways
Expenditure
on Seminar,
Conference,
Workshop,
Exhibition
Sponsorship Any
Outsourcing
Activities
Miscellaneous
Expenses
under the
Rules
Total
Electronic
Media
Print
Media
Local Foreign
(1) (2) (3) (4) (5) (6) (7) (8) (9) (10)
Signature & Stamp
[No. F.13-2/2017-DD(PS)]
DR. ABDUR RASHID,
Director (Pharmacy Services).](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-182-320.jpg)








![Page 3 of 14
THE PHARMACY ACT, 1967
ACT No. XI OF 1967
[20th
June 1967]
An Act to establish Pharmacy Councils to regulate the practice of pharmacy
WHEREAS it is expedient to establish Pharmacy Councils to regulate the practice of
pharmacy and to provide for matters connected therewith and incidental thereto ;
AND WHEREAS the national interest of Pakistan in relation to the achievement of
uniformity within the meaning of clause (2) of Article 131 of the Constitution requires Central
legislation in the matter ;
It is hereby enacted as follows:⸻
1. Short title, extent and commencement.⸻(1) This Act may be called the Pharmacy Act,
1967.
(2) It extends to the whole of Pakistan.
(3) It shall come into force at once.
2. Definitions.⸻In this Act, unless there is anything repugnant in the subject or
context,⸻
(a) “approved” means approved under section 18 or, as the case may be, section 19;
(b) “Central Council” means the Pharmacy Council of Pakistan established under
section 3;
(c) “Council” means a Pharmacy Council established under section 3 ;
(d) “Medical Institution” means an institution whose medical qualifications are
recognised under the 1
[Medical and Dental Council Ordinance, 1962 (XXX of
1962)];
2
[(e) ‘Pakistan Pharmacists Association’ means the association registered under the
Societies Registration Act, 1860 (XXI of 1860), and known at the
commencement of the Pharmacy (Amendment) Act, 1973, by that name ;
(f) ‘Pharmacist’ means a person who, is registered under section 24 in Register A
or Register B;
(g) ‘Provincial Council’ means the Pharmacy Council of a Province established under
section 3.].
1
Subs. by the Federal Laws (Revision and Declaration) Ordinance No. XXVII of 1981, s. 3 and Sch., II.
2
Subs. by Act No. XXII of 1973, s. 2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-192-320.jpg)
![Page 4 of 14
3. Establishment of Pharmacy Councils.⸻(1) Within a period of one year from the
commencement of this Act,—
(a) The 1
[Federal Government] shall, by notification in the official Gazette,
establish a Central Pharmacy Council to be known by the name of the
Pharmacy Council of Pakistan ; and
2
[(b) each Provincial Government shall, in like manner, establish a Provincial
Pharmacy Council to be known by the name of the Province concerned.”.
(2) Each of the Pharmacy Councils established under sub-section (1) shall be a body
corporate having perpetual succession and common seal, with power, among others, to acquire, hold
and dispose of property, and shall by its name sue and be sued.
4. Composition of Central Council.⸻(1) The Central Council shall, subject to the
provisions of sub-section (2), consist of the following members, namely:—
(a) the Director-General of Health, Government of Pakistan, ex-officio, who shall,
unless the 1
[Federal Government] appoints any other officer to be the President,
also be the President of the Council ;
(b) the officer, if any, appointed under clause (a) to be the President of the Council ;
2
“[(c) eight persons, to be nominated by the Federal Government, out of whom one
from each Province shall be nominated in consultation with the Provincial
Government concerned, one shall be a teacher of pharmaceutics and one a
teacher of pharmaceutical chemistry ;
(d) one person from each Province, to be nominated by the Federal Government, so
far as may be, in consultation with the Provincial Council concerned ;
(e) one person, to be nominated by the Federal Government in consultation with the
Pakistan Pharmacists Association ; and
(f) the Drugs Controller, Government of Pakistan.” ; and
(2) The 1
[Federal Government] may, by notification in the official Gazette, increase or
decrease the number of persons to be nominated by it under clause (c) of sub-section (1).2
* * :
Provided that the decrease in the number of members shall not affect the continuance in
office of, and the performance of functions by, any member until the expiry of his term.
5. Composition of the Provincial Council.⸻(1) A Provincial Council shall, subject to the
provisions of sub-section (2), consist of the following members, namely :—
1
Subs. by F.A.O., 1975, Art. 2 and Table.
2
Subs. and omitted by Act No. XXII of 1973, ss. 3 and 4.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-193-320.jpg)
![Page 5 of 14
2
“(a) The Secretaries to the Provincial Governments in the Health Department, ex-
officio, who shall, unless the Provincial Government appoints any other officer
to be the President, also be the Presidents of the respective Councils ” ; and
(b) the officer, if any, appointed under clause (a) to be the President of the
Council ;
2
“(c) five persons to be nominated by the Provincial Government, of whom one
shall be an officer of that Government ; and
(d) one person to be nominated by the Provincial Branch of the Pakistan
Pharmacists Association “.
(2) The Provincial Government may, by notification in the official Gazette, increase or
decrease the number of persons to be nominated by it under clause (c) of sub-section (1) :
Provided that the decrease in the number of members shall not affect the continuance in office
of, and the performance of functions by, any member until the expiry of his term.
2
[6. Disqualification for membership.⸻A person, other than a professor of medical
institution or a pharmacy institution or an officer of the Provincial Government nominated under
clause (c) of sub-section (1) of section 5, shall not be eligible for nomination as a member of the
Council unless he is a pharmacist registered in Register A :
3
[* * * * * * *]
7. Publication of names.⸻The 1
[Federal Government] or, as the case may be, the Provincial
Government shall publish in the official Gazette the names or the official titles of the members of the
Council.
8. Term of office.⸻(1) Subject to the provisions of sub-section (2), a member other than an
ex-officio member shall hold office for a period of three years commencing on the day on which he
assumes office and shall be eligible for re-nomination :
Provided that notwithstanding the expiry of his term a member shall continue to function
until his successor assumes office.
(2) Where the 1
[Federal Government] or, as the case may be, the Provincial Government,
upon the recommendation of a majority of the members of the Council, is satisfied that a member of
the Council is negligent in the discharge of his duties or is guilty of any unprofessional or
dishonorable conduct or is otherwise not competent to perform the functions of a member, it may, by
notification in the official Gazette, remove such member ; and upon the publication of such
notification the seat of the member shall become vacant.
9. Filling of casual vacancy.⸻A casual vacancy in the office of a member shall be filled for
the remainder of the term of such member, not being less than six months, by nominating another
person in his place, in the same manner in which such member was nominated.
1
Subs. by F. A. O., 1975, Art. 2 and Table.
2
Subs. by Act No. XXII of 1973, ss. 5 and 6.
3
Omitted by the Federal Laws (Revision and Declaration) Ordinance No. XXVII of 1981, s. 3 and Sch. II.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-194-320.jpg)
![Page 6 of 14
10. Vacancy, etc., not to invalidate the proceedings of a Council.⸻No act or proceedings
of a Council shall be invalid merely on the ground of the existence of any vacancy in, or any defect
in the composition of the Council.
11. Election of Vice-President.⸻(1) A Council shall every year elect one of its members to
be the Vice-President of the Council and the Vice-President so elected shall hold office for a period
of one year and shall be eligible for re-election :
Provided that a Vice-President shall, notwithstanding the expiry of his term, continue to
function until his successor is elected.
(2) The Vice-President shall perform such functions as may be entrusted to him by the
Council and, in the absence of the President, also the functions of the President.
12. Committees of a Council.⸻(1) A Council may constitute such committees as it deems
fit for the purpose of advising and assisting it in the performance of its functions.
(2) A committee constituted under sub-section (1) may co-opt as its member any person
whose assistance or advice it may consider necessary for the efficient performance of its functions.
13. Meetings of a Council.⸻(1) A Council shall meet at such time and place, and a meeting
of the Council shall be summoned and conducted in such manner, as may be laid down by its bye-
laws :
Provided that, until such bye-laws are made, the President of the Council may, by notice
addressed to each member, summon and conduct a meeting at such time and place and in
such manner as he may deem expedient.
(2) The President and, in his absence, the Vice-President shall preside at every meeting of the
2
[Council] and, in the absence of both the President and the Vice-President, the members present
shall elect one amongst them to preside.
2
“[(3) The quorum for a meeting of the Council shall be one-third of the total number of
members, a fraction being counted as one. ”.
14. Annual report.⸻As soon as may be after the close of every year, the Central Council
shall submit to the 1
[Federal Government] and a Provincial Council to the Provincial Government,
an annual report giving an account of its proceedings together with a statement of moneys received
and expenses incurred by it during that year.
15. Appointment of Secretary, officers and staff of the Council.⸻(1) A Council shall,
with the approval in the case of the Central Council of the 1
[Federal Government] and in the case of
a Provincial Council of the Provincial Government, appoint a Secretary from amongst persons
eligible for registration as pharmacists 2
[in Register A] on such terms and conditions as it may deem
fit.
(2) The Council may also appoint such officers and staff as may be necessary for the efficient
performance of its functions.
1
Subs. by F.A.O., 1975, Art. 2 and Table.
2
Subs. and ins. by Act No. XXII of 1973, ss. 7 and 8.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-195-320.jpg)
![Page 7 of 14
16. Finances.⸻(1) The funds of the Central Council shall consist of such moneys as may be
placed at its disposal by the 1
[Federal Government].
(2) The funds of a Provincial Council shall consist of the fees received by it under this Act
and of such moneys as may be placed at its disposal by the Provincial Government.
17. Functions of the Central Council.⸻(1) The functions of the Central Council shall be—
(a) to approve examinations in pharmacy for the purpose of qualifying persons for
registration as pharmacists ;
(b) to prescribe the subjects in which approved examinations shall be held ;
(c) to approve the courses of study and practical training in pharmacy for the
purpose of admission to approved examinations ;
(d) to prescribe the conditions and procedure for admission of candidates to an
approved examination ;
(e) to lay down the standard of teaching to be maintained by institutions
conducting the approved courses of study ;
(f) to prescribe the equipment and facilities to be made available to the students ;
(g) to recognise degree or diplomas in pharmacy for the purpose of registration as
pharmacists ;
(h) to cause inspection of institutions which conduct any courses of study in
pharmacy and of the teachings imparted and examinations held by them ; and
(i) to do such other acts and things at it may be empowered or required to do by
or under this Act.
(2) The Central Council, with the previous approval of the 1
[Federal Government] may, by
notification in the official Gazette, make regulations for the purposes of sub-section (1).
18. Approval of examinations.⸻(1) Any institution or authority, including a Provincial
Council, which holds an examination in pharmacy, may apply to the Central Council for approval of
the examination for the purpose of qualifying a person for registration as a pharmacist under this Act.
(2) The Central Council, if it is satisfied after such enquiry as it may think fit that the
examination for the approval of which an application has been made under sub-section (1) is in
conformity with this Act and the regulations, shall approve the examination and, by notification in
the official Gazette, declare it to be an approved examination for the purpose of qualifying a person
for registration as a pharmacist under this Act.
19. Approval of courses of study.⸻(1) Any institution or authority which conducts a course
of study in pharmacy may apply to the Central Council for approval of such course of study for the
purpose of admission to an approved examination.
1
Subs. by F.A.O., 1975, Art. 2 and Table.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-196-320.jpg)
![Page 8 of 14
(2) The Central Council, if it is satisfied after such enquiry as it may think fit that the course
of study for the approval of which an application has been made under sub-section (1) is in
conformity with this Act and the regulations, shall submit the application together with its
recommendation to the 1
[Federal Government] and shall, upon the approval of the course of study by
the 1
[Federal Government], declare it, by notification in the official Gazette, to be an approved
course of study for the purpose of admission to an approved examination.
20. Furnishing of information.⸻Every institution or authority which applies for the
approval of an examination under section 18 or of a course of study under section 19, or holds an
approved examination, or conducts an approved course of study, shall furnish to the Central Council
such information as the Council may, from time to time, require relating to—
(a) the course of study conducted and training given ;
(b) the examination held ;
(c) the ages at which the students may undergo the course of study ;
(d) the equipment and facilities provided for the students ; and
(e) matters generally pertinent to the course of study, training and examinations and
standard of teaching.
21. Inspectors.⸻(1) The Central Council may appoint such Inspectors for the inspection of
institutions as it may consider necessary.
(2) An Inspector appointed under sub-section (1) may, if he is so authorised in writing by
the President of the Council,—
(a) inspect any institution which holds an approved examination or conducts an
approved course of study and may attend any such examination held by such
institution ;
(b) inspect any institution which has applied for the approval of the examination held,
or course of study conducted, by it and attend any examination held by such
institution.
(3) An Inspector who attends any examination shall not interfere with the conduct thereof but
shall submit to the Central Council a report on the sufficiency or otherwise of such examination and
on any other matter in regard to which the Central Council may require him to report.
22. Withdrawal of approval.⸻(1) Where, upon a report by an Inspector, it appears to the
Central Council that an approved course of study or an approved examination does not continue to be
in conformity with this Act and the regulations, the Central Council shall give notice to the
institution or authority concerned calling upon it to explain in writing why the approval of its course
of study or examination should not be withdrawn.
(2) The institution or authority to whom a notice has been given under sub-section (1) shall,
within sixty days from the receipt of such notice, comply with the notice and may also make such
representation to the Central Council, through the Provincial Government, as it may wish to make.
1
Subs. by F.A.O., 1975 Art. 2 and Tabl](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-197-320.jpg)

![Page 10 of 14
(h) date on which registered ; and
(i) such other particulars as may be prescribed by bye-laws.
25. Qualifications for registration as a pharmacist or as an apprentice in
pharmacy.⸻(1) The following persons shall, subject to the provision of sub-section (3), be
qualified for registration as pharmacists under this Act, namely :—
(a) persons who hold a degree in pharmacy conferred by a University or an institution
affiliated thereto, where the degree is recognised by the Central Council ;
(b) persons who hold a diploma in pharmacy granted by any institution recognised by
the Central Council ; and
(c) persons who pass the examination in pharmacy held by a Provincial Council 1
[*]
2
[* * * * * * *]
2
“ [(1A) Subject to the provisions of sub-section (3), during the period of one year from the
commencement of the Pharmacy (Amendment) Act, 1973, a person who was, on the 19th
day of
June, 1972, to be deemed to be qualified for registration as a pharmacist shall be deemed to be so
qualified. ” ; and
2
[(2) The following persons shall, subject to the provisions of sub-section (3), be qualified to
be registered as an apprentice in pharmacy, namely :—
(i) an Inspector of Drugs and a Government Analyst appointed under the 2
[Drugs
Act, 1976 (XXXI of 1976)], if not otherwise eligible for registration ;
(ii) a person certified by a Government Hospital to be a qualified compounder and
dispenser ;
(iii) a person who has been taken as a student or apprentice in pharmacy by, and
produces a certificate to that effect from, a pharmacist registered in Register A
and approved for the purpose, by notification in the official Gazette, by the
Provincial Government ; and
(iv) a person who is a qualified person within the meaning of rule 65 of the West
Pakistan Drugs Rules, 1958, if not otherwise eligible for registration.].
(3) No person shall be qualified for registration as a pharmacist or as an apprentice in
pharmacy⸻
(a) if he is of unsound mind and stands so declared by a court ; or
(b) if he has been convicted by a court of any offence which in the opinion of the
Provincial Council involves moral turpitude.
26. Procedure for registration.⸻(1) As soon as may be after the opening of the Registers
under section 24, the Provincial Council shall, by notification in the official Gazette, invite applications
from persons desirous of being registered as pharmacists or as apprentices in pharmacy.
1
Subs., ins., and omitted by Act No. XXII of 1973, s. 10.
2
Subs. by the Federal Law (Revision and Declaration) Ordinance No. XXII of 1981, s. 3 and Sch. II.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-199-320.jpg)
![Page 11 of 14
(2) An application for registration shall contain such particulars and be made in such form as
may be specified by the Provincial Council and shall be accompanied by such fee as may be
prescribed by the bye-laws.
(3) The Provincial Council shall examine every application received by it and, if it is satisfied
that the applicant is qualified for registration under section 25, direct the entry of the name of the
applicant in the appropriate Register.
(4) The Provincial Council shall, if it rejects the application of any person, inform the applicant
in writing of such rejection within ninety days from the date of receipt of the application, and the
applicant may within sixty days of the receipt of the information appeal against such rejection to the
Provincial Government whose decision thereon shall be final.
(5) Failure to inform the applicant of the rejection within the period specified in sub-section (4)
shall be treated as acceptance of the application for registration.
27. Certificate of registration.⸻(1) The Provincial Council shall issue a certificate of
registration to a person who has been registered under section 26.
(2) A certificate of registration issued under sub-section (1) shall bear a number and the official
seal of the Council and be signed by its President and the Secretary and shall contain the following,
namely :—
(a) a passport size photograph of the person registered ;
(b) the full signature of the person registered ; and
(c) an endorsement of any mark of identification of the person registered.
(3) A copy of the certificate with all the particulars specified in sub-section (2) shall be kept in
the official records of the Council.
(4) A person to whom a certificate of registration has been issued may, if the original is lost,
defaced or mutilated or for any other reason, obtain a duplicate thereof on payment of the same fee
as was paid for the original.
28. Revocation of certificate.⸻(1) The Provincial Council may, after giving the person
concerned an opportunity to make representation and of being heard, revoke the certificate of
registration issued to him, if such person⸻
(a) incurs any disqualification specified in sub-section (3) of section 25 ; or
(b) contravenes any of the provisions of the Poisons Act, 1919 (XII of 1919), the
Dangerous Drugs Act, 1930 (II of 1930), the 1
[Drugs Act, 1976 (XXXI of 1976)],
or this Act or of the rules made under any of those Acts ; or
(c) fails or neglects to comply with any directive in respect of the profession of a
pharmacist with the 2
[Federal Government] or the Provincial Government may,
from time to time, issue ; or
1
Subs. by the Federal Laws (Revision and Declaration) Ordinance, 1981, s. 3 and Sch., II.
2
Subs. by F. A. O., 1975, Art. 2 and Table.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-200-320.jpg)
![Page 12 of 14
(d) is guilty of such professional misconduct as may be laid down by the Provincial
Council in this behalf.
(2) Where any certificate of registration is revoked under sub-section (1), the name of the person
whose certificate has been so revoked shall, after he has been given a notice in writing of such
revocation, be struck off the register in which his name was entered and his registration shall thereupon
stand cancelled.
(3) The Provincial Council may, of its own motion, and shall, upon an application made in this
behalf within thirty days of the receipt of the notice under sub-section (2) by the person concerned,
review its decision to revoke a certificate of registration ; and the decision of the Council upon such
review shall be final.
29. Examination for registration as pharmacists.⸻(1) For the purpose of registration as
pharmacists, the Provincial Council shall, after giving notice in this behalf hold examinations twice in
every year.
(2) An examination under sub-section (1) shall be held 1
[at such place in a Province as the
Provincial Council may decide”].
(3) Notice of an examination shall be published for a continuous period of not less than one
week in at least one newspaper in English and one newspaper in the local language, each having wide
circulation in the Province.
(4) Every application for admission to an examination shall be made in such manner and in such
form as may be specified by the Provincial Council and shall be accompanied by⸻
(a) such fee as may be prescribed by the bye-laws ;
(b) a certificate of good moral character from a respectable person ; and
(c) such other papers or particulars as may be required by the Provincial Council.
30. Qualifications for admission to an examination.⸻An applicant for admission to an
examination under section 29,⸻
(a) shall not be below seventeen years of age on the date fixed for the examination
;
(b) must have passed the matriculation examination or an equivalent Higher
Secondary or Senior Cambridge examination with general science as one of
the subjects,
1
[* * *] ; and
(c) must have been registered as an apprentice in pharmacy for a period of not
less than two years before the date fixed for the examination :
1
Subs. by Act No. XXII of 1973, s. 11.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-201-320.jpg)
![Page 13 of 14
Provided that clause (c) shall not apply during any period during which registration of
apprentices in pharmacy remains discontinued under the proviso to sub-section (1) of
Section 24 and the period of two years thereafter 1
[:]
1
[Provided further that, notwithstanding anything contained in this Act, it shall not be
necessary for any apprentice in pharmacy to attend any regular classes or to complete any number of
days or lectures at any institution, for the purpose of being qualified to be admitted to an
examination under Section 29.].
31. Prohibition of practice without registration.⸻(1) Subject to the provisions of sub-
section (4), no person shall, after the expiry of five years from the commencement of this Act or such
later date as the 2
[Federal Government] may, by notification in the official Gazette, specify in this
behalf, practice as a pharmacist unless he is a registered pharmacist and displays his certificate of
registration in a conspicuous place within the premises in which he so practices.
(2) Whoever employs any pharmacist for the purpose of any business in pharmacy shall
cause the certificate of registration of the pharmacist so employed to be displayed in a conspicuous
place within the premises in which such business is carried on.
1
[(3) Whoever contravenes the provisions of sub-section (1) or sub-section (2) shall be
punishable, with imprisonment of either description for a term which may extend to six months, or
with fine which may extend to one thousand rupees, or with both.” ; and
(4) Nothing in sub-section (1) shall apply to⸻
(a) a registered medical practitioner as defined in the 3
[Medical and Dental
Council Ordinance, 1962], or a person authorized to prescribe antibiotic and
dangerous drugs under the Allopathic System (Prevention of Misuse)
Ordinance, 1962 (LXV of 1962), who dispenses medicine to his own patients
or serves his own prescriptions ;
(b) a person who deals in non-poisonous household remedies in original and
unopened container at any store or place or prepares non-poisonous household
remedies in accordance with the rules made under the 1
[Drugs Act, 1940
(XXIII of 1940)] ;
(c) a person who manufactures, sells or distributes drugs and medicines which fall
exclusively under the unani, ayurvedic, biochemic or homeopathic system of
medicine ;
(d) a person engaged as a health or veterinary technician in a Government hospital
or institution ; 2
[*]
(e) a foreign pharmacist who is engaged, with the approval of the Central
Council, for the purposes of consultation, advice or instruction 2
[; and]
1
Omitted, subs. and added by Act No. XXII of 1973 ss. 12 and 13.
2
Subs. by F.A.O., 1975, Art. 2 and Table.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-202-320.jpg)
![Page 14 of 14
“ 2
[(f) an apprentice in pharmacy, during the period of four years from the
commencement of the Pharmacy (Amendment) Act, 1973, or during such
further period as the Federal Government may, by notification in the official
Gazette, specify in this behalf.].
32. Cognizance of offences, etc.⸻No court shall take cognizance of an offence under this
Act except upon a complaint in writing made by an Inspector appointed under the 1
[Drugs Act,
1976], or an officer specially empowered in this behalf by the Provincial Government.
33. Indemnity.⸻No suit, prosecution or other legal proceeding shall lie against any person
for anything which is in good faith done or intended to be done under this Act.
34. Power to make bye-laws.⸻(1) A Council may with the previous approval, in the case of
the Central Council of the 1
[Federal Government], and in the case of a Provincial Council of the
Provincial Government, make bye-laws for carrying out the purposes of this Act.
(2) In particular and without prejudice to the generality of the foregoing power, such bye-
laws may provide for all or any of the following matters, namely :⸻
(a) the procedure for the meetings of the Council and of its committees ;
(b) the management of the property of the Council ;
(c) maintenance and audit of the accounts of the Council ;
(d) the procedure for election of the Vice-President ;
(e) the powers and duties of the President, Vice-President and other members of
the Council ;
(f) the terms and conditions of service of the Secretary and other officers and
staff of the Council ;
(g) fees to be prescribed under this Act ; and
(h) such other matters as are required by this Act to be provided for by bye-laws
or are considered necessary for the efficient performance of the functions of
the Council.
(3) Until such time as the bye-laws are made, the President of the Council may issue such
instructions as he may consider necessary to regulate all or any of the matters specified in sub-
section (2) ; and any such instructions shall stand rescinded upon the making of bye-laws by the
Council.
__________
Date: 13-09-2024
1
Subs. by F.A.O., 1975, Art. 2 and Table.
2
Omitted and added by Act No. XXII of 1973 s. 13.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-203-320.jpg)





![Page 5 of 50
THE CONTROL OF NARCOTIC SUBSTANCES ACT, 1997
1ACT NO. XXV OF 1997
An Act to consolidate and amend the laws relating to narcotic drugs and psychotropic substances
WHEREAS it is expedient to consolidate and amend the laws relating to narcotics drugs,
psychotropic substances, 2
[controlled substances] and control the production, processing and
trafficking of such drugs and 2
[to provide for forfeiture of property derived from or used in illicit
traffic in narcotic drugs, psychotropic substances and controlled substances and to implement the
provisions of the international conventions on narcotic drugs, psychotropic substances and
controlled substances].
AND WHEREAS it is expedient to regulate the treatment and rehabilitation of narcotic addicts
and for matters connected therewith and incidental thereto;
It is hereby enacted as follows :___
CHAPTER I
PRELIMINARY
1. Short title and commencement.__(1) This Act may be called the Control of Narcotic
Substances Act, 1997.
(2) It extends to the whole of Pakistan.
(3) It shall come into force at once.
2. Definitions.___In this Act, unless there is anything repugnant in the subject or context,___
(a) “addict” means a person physically or mentally dependent on any narcotic
drug or psychotropic substance or a person who habitually uses narcotic
drugs or psychotropic substances;
(b) “assets” means any property owned, controlled or belonging to an accused,
whether directly or indirectly, or in the name of his spouse or relatives
or associates whether within or without Pakistan for which they cannot
reasonably account ;
(c) “associate”, in relation to an accused, means__
(i) any individual who is, or has, at the relevant time been
ordinarily residing in the residential premises including out-houses
and servant-quarters of an accused;
(ii) any individual who, is or has, at the relevant time been
managing the affairs or keeping the accounts of an accused ;
1
This Act shall apply to FATA, vide S.R.O. No. 1295(I)/98, dated 16-11-1998.
2
Ins. and subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s. 2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-209-320.jpg)
![Page 6 of 50
(iii) any association of persons, body of individuals, firm or private
limited company within the meaning of 1
[Companies Act, 2017 (XIX
of 2017) and the Limited Liability Partnership Act, 2017 (XV of
2017)], of which an accused is, or has, at the relevant time been a
member, partner or director;
(iv) any individual who is, or has, been at the relevant time a member,
partner or director of any association of persons, body of individuals,
firm or a private limited company referred to in sub-clause (iii);
(v) a trustee of any trust created by an accused; or
(vi) where the Special Court, for reasons to be recorded, considers that
any property of an accused is held on his behalf by any other person,
such other person;
(d) “cannabis (hemp)” means___
(i) cannabis resin (charas) that is, the separated resin, whether crude
or purified, obtained from the cannabis plant and also includes
concentrated preparation and resin known as hashish oil or liquid
hashish;
(ii) the flowering or fruiting tops of the cannabis plant (excluding the
seed and leaves when not accompanied by the tops) from which the
resin has not been extracted, by whatever name they may be
designated or known 1
[and include all forms known as bhang, siddhi
or ganja]; and
(iii) any mixture with or without neutral materials of any of the above
forms of cannabis or any drink prepared therefrom;
(e) “cannabis plant” means any plant of the genus cannabis;
(f) “coca bush” means the plant of any species of the genus Erythroxylon;
(g) “coca derivative” means__
(i) crude cocaine, that is, any extract of coca leaf which can be used,
directly or indirectly for the manufacture or production of cocaine;
(ii) ecgonine, that is, leave-ecgonine having the chemical formula
C9H15NO3H2O and all chemical derivatives of leavo-ecgonine
including benzoylecgonine from which it can be recovered;
(iii) cocaine, that is, methyl-benzoyl-leave-ecgonine having the chemical
formula C17H21NO4 and its salts; and
(iv) all preparations containing more than 0.1 per cent of cocaine;
1
Subs. and Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s. 3.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-210-320.jpg)
![Page 7 of 50
(h) “coca leaf” means__
(i) the leave of the coca bush except a leaf from which all ecgonine,
cocaine or any other ecgonine alkaloids have been removed;
(ii) any mixture thereof, with or without neutral material, but does not
include any preparation containing not more than 0.1 per cent of
cocaine;
(j) “controlled delivery” means the technique of allowing illicit or suspect
consignments of narcotic drugs, psychotropic substances or chemical
precursors to pass out of, through or into Pakistan, with the knowledge and
under the supervision of the Federal Government with a view to
identifying persons involved in the commission of offences cognizable under
this Act;
(k) “controlled substance” means any substance which may be used for the
production or manufacture of narcotic drugs or psychotropic
substance 1
[or which is declared to be a controlled substance and given in the
Schedule-II pursuant to the provision of any international convention, and by
notification in the official Gazette by the divison concerned,];
(l) “conveyance” means a conveyance of any description whatsoever and
includes, any aircraft, vehicle, vessel, railways or animal;
(m) “Director-General” means Director-General of the Anti-Narcotics Force or
anyother officer appointed by the Federal Government to perform the duties
and functions of the Director-General under this Act; and
(n) “foreign court” means a court of competent jurisdiction of a foreign
country recognised by the Federal-Government from time to time;
(o) “freezing” means prohibiting by an order made by the Special Court or an
officer authorised under this Act the transfer, conversion, disposal or
movement of any assets and includes the holding, controlling, assuming
custody or managing any assets in pursuance of such order and, in the case
of assets which are perishable the disposal thereof;
1
[(oa) “illicit traffic” in the relation to narcotic drugs, psychotropic substances or
controlled substances means __
(i) cultivating any coca plant or gathering any portion of cocoa plant;
(ii) cultivating the opium poppy or any cannabis plant or gathering in any
portion of opium poppy or cannabis plant;
1
Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s. 3.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-211-320.jpg)
![Page 8 of 50
(iii) engaging in the production, manufacture, possession, sale, purchase,
transportation, warehousing, concealment, use or consumption, import
into Pakistan, export from Pakistan or transship any narcotic drugs or
psychotropic substances or controlled substances;
(iv) dealing in any activities in narcotic drugs or psychotropic substances or
controlled substances other than those referred to in sub-clauses (i) to
(iii);
(v) handling or letting out any premises for the carrying on of any of the
activities referred to in sub-clauses (i) to (iv);
(vi) financing directly or indirectly any of the aforementioned activities;
(vii) abetting or conspiring in the furtherance of or in support of doing any of
the aforementioned activities; or
(viii) harboring persons engaged in any of the aforementioned activities.;
(ob) “international convention” means__
(i) the Single Convention on Narcotic Drugs done at New York on the
30th
March, 1961, as amended by the 1972 Protocol done at Geneva on
the 25th
March, 1972;
(ii) the Convention Against Psychotropic Substances done at Vienna on
the 21st
February, 1971;
(iii) the United Nations Convention Against Illicit Traffic in Narcotic
Drugs and Psychotropic Substances done at Vienna on the 20th
December, 1988; and
(iv) any other international convention to which Pakistan may become
party in future relating in whole or in part to the control of drugs of
abuse, controlled chemicals or controlled equipments;]
(p) “manufacture”, in relation to narcotic drugs or psychotropic substances,
includes__
(i) all processes by which such drugs or substances may be
obtained;
(ii) refining of such drugs or substances;
(iii) transformation of such drugs or substances; and
(iv) making or preparing such drugs or substances;
(q) “manufactured drug” includes__
(i) all coca derivatives, medicinal hemp, opium derivatives, cannabis
in any form and any mixture of stalks and flowering or fruiting
tops of the Indian hemp plant (cannabis sativa L.), Acetic Anhydride;
and](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-212-320.jpg)

![Page 10 of 50
(y) “prescribed” means prescribed by rules made under this Act;
(z) “property” includes__
(i) all forms of property, whether corporeal or incorporeal, movable
or immovable, tangible or intangible, real estate or personal
propertyof every description;
(ii) property used to commit, or to abet the commission of, an offence
punishable under this Act;
(iii) all kinds of shares or interest in any corporate body, company, firm,
business concern, society or fund; and
(iv) all documents of title to land, goods or property wherever situated,
money or valuable security issued by the Government;
(za) “psychotropic substance” means the substances, specified in the 1
[Schedule-I]
to this Act and such substances as the Federal Government may, by
notification in the official Gazette, declare to be a psychotropic substance;
(zb) “relative”, in relation to an accused, means the spouse or any lineal
descendant of the accused and includes any other person holding property
for or on his behalf;
(zc) “Special Court” means the Special Court established under section 46 or any
other court empowered to exercise the powers of the Special Court under
this Act; and
(zd) “tracing” means the finding out the true nature, source, disposition, movement
or ownership of assets and includes determining the movement or
conversion of assets by any means, and “trace” shall be construed
accordingly.
3. Calculation of percentages in liquid preparations.___ The Federal Government may
make rules prescribing the methods by which percentages in the case of liquid preparations shall be
calculated for the purposes of clauses (g), (h), (t) and (u) of section 2 :
Provided that, unless and until such rules are made, such percentages shall be calculated on
the basis that a preparation containing one per cent of a substance means a preparation in which
one gram of the substance, if a solid, or one milliliter of the substance, if a liquid, is contained in
every one hundred milliliters of the preparation, and so in proportion for any greater or less
percentage.
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s. 3.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-214-320.jpg)
![Page 11 of 50
CHAPTER II
PROHIBITION AND PUNISHMENT
4. Prohibition of cultivation of narcotic plants.__ No one shall cultivate 1
[or let his land
for cultivation or give possession for cultivation of] any cannabis plant, coca bush or opium poppy,
or gather any portion of a cannabis plant, coca bush or opium poppy:
Provided that the Federal Government or a Provincial Government authorised by the
Federal Government may, subject to such conditions as it may prescribe, permit under a licence
cultivation or gathering of any such narcotic plant or any portion thereof exclusively for
medical, scientific or industrial purposes.
5. Punishment for contravention of section 4.__ Whoever contravenes the provisions of
section 4 shall be punishable with imprisonment which may extend to seven years 1
[but shall not be
less than one year and also be liable to fine].
6. Prohibition of possession of narcotic drugs, etc.__ No one shall produce, manufacture,
extract, prepare, possess, offer for sale, sell, purchase, distribute, deliver on any terms whatsoever,
transport, despatch, any narcotic drug, psychotropic substance or controlled substance, except for
medical, scientific or industrial purposes in the manner and subject to such conditions as may be
specified by or under this Act or any other law for the time being in force.
7. Prohibition of import or export of narcotic drugs, etc.__ (1) No one shall__
(a) import into Pakistan;
(b) export from Pakistan;
(c) transport within Pakistan; or
(d) transship.
any narcotic drug, psychotropic substance or controlled substance, save in accordance with rules
made under sub-section (2) and in accordance with the conditions of any licence, permit or
authorization for that purpose which may be required to be obtained under those rules.
(2) The Federal Government may make rules permitting and regulating the import into and
export from Pakistan, transport within Pakistan and transhipment of narcotic drugs, psychotropic
substances or controlled substances, and such rules may prescribe the ports or places at which any
kind of narcotic drug, psychotropic substance or controlled substance may be imported, exported,
transported within Pakistan or transhipped, the form and conditions of licence, permit or authorities
by which such licences, permits or authorization may be granted, the fees that may be charged
therefor, any other matter required to have effective control of the Federal Government over such
import, export, transportation and transshipment.
8. Prohibition on trafficking or financing the trafficking of narcotic drugs etc.__
No one shall—
(a) organize, manage, traffic in, or finance the import, transport, manufacturing or
trafficking of, narcotic drugs, psychotropic substances or controlled
substances; or
(b) use violence or arms for committing or attempt to commit an offence
punishable under this Act.
1
Ins. and subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) ss.4 - 5.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-215-320.jpg)

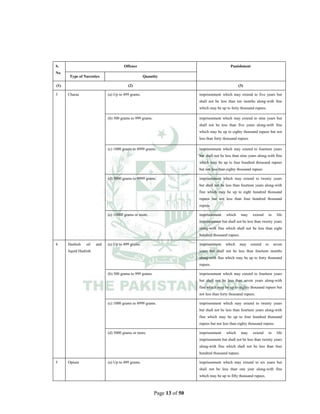

![Page 15 of 50
S.
No
Offence Punishment
Type of Narcotics Quantity
(1) (2) (3)
(f) 6000 grams or more.
1
[* * *] imprisonment which shall not be less than life
along-with fine which may extend to two million but
shall not be less than one and half million rupees.
7 Cocaine (a) Up to 99 grams. imprisonment which may extend to seven
years but shall not be less than eighteen months
along-with fine up to fifty thousand rupees.
(b) 100 grams to 999 grams. imprisonment which may extend to fifteen years but
shall not be less than seven years along-with fine
which may be up to five hundred thousand rupees
but not less than fifty thousand rupees.
(c) 1000 grams to 4999 grams. imprisonment which may extend to twenty years but
shall not be less than fifteen years along-with fine
which may be up to two million and five hundred
thousand rupees but not less than five hundred
thousand rupees.
(d) 5000 grams or more.
1
[* * *] imprisonment for life but imprisonment shall
not be less than twenty years along-with fine which
shall not be less than two million and five hundred
thousand rupees.
Provided that if an offence is committed relating to narcotic drug inside or near a school,
college, university, educational setting or any other educational institution maximum punishment
provided for that offence shall be awarded:
Provided further that if any person who has previously been convicted for any offence under
this Act is subsequently convicted for the offence relating to narcotic drug, he shall be convicted
with maximum punishment provided for that offence.
(2) Whoever contravenes the provisions of sections 6, 7 and 8 regarding psychotropic
substances shall be punished with punishment as given in column (3) of the TABLE below with
regard to quantity of psychotropic substances given in column (2) thereof, namely:___
TABLE
Sr.
No
Offence with regard to quantity of psychotropic substance Punishment
(1) (2) (3)
1 Up to 20 grams. Imprisonment which may extend to one year but
shall not be less than two months along-with fine
which may be up to fifty thousand rupees.
1
Omitted by Act XXXVIII of 2023, s.2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-219-320.jpg)
![Page 16 of 50
Sr.
No
Offence with regard to quantity of psychotropic substance Punishment
(1) (2) (3)
2 More than 20 grams and up to 50 grams. Imprisonment which may extend to two years but
shall not be less than one year along-with fine which
may be up to one hundred thousand rupees.
3 More than 50-grams and up to 100 grams. Imprisonment which may extend to three years but
shall not be less than two years along-with fine
which may be up to two hundred thousand rupees.
4 More than 100-grams and up to 500 grams. Imprisonment which may extend to five years but
shall not be less than three years along-with fine
which may be up to four hundred thousand rupees.
5 More than 500-grams and up to one kilo grams. Imprisonment which may extend to seven years but
shall not be less than five years along-with fine
which may be up to eight hundred thousand rupees.
6 More than one kilo grams and up to two kilo grams. Imprisonment which may extend to ten years but
shall not be less than seven years along-with fine
which may be up to twelve hundred thousand rupees.
7 More than two kilo grams and up to three kilo grams. Imprisonment which may extend to fourteen years
but shall not be less than ten years along-with fine
which may be up to sixteen hundred thousand
rupees.
8 More than three kilo grams and up to four kilo grams. Imprisonment which may extend to twenty years but
shall not be less than fourteen years along-with fine
which may be up to eighteen hundred thousand
rupees.
9 Exceeding four kilo grams. Imprisonment which shall not be less than life
imprisonment along-with fine which shall not be less
than two million rupees.
Provided that if any offence is committed relating to psychotropic substance inside or near a
school, college, university, educational setting or any other educational institution, he shall be
punishable with maximum punishment provided for that offence:
Provided further that if any person who has previously been convicted for any offence under
this Act is subsequently convicted for the offence relating to psychotropic substance and quantity
does not exceed two kilograms than he shall be convicted with maximum punishment provided for
that offence:
Provided also that if the quantity of psychotropic substance in subsequent offence exceeds
two kilograms, the punishment shall not be less than life imprisonment.
Provided furher that if recovered psychotropic substance is methamphetamine (ICE) given at
serial number 47 of the Schedule-I to this Act and quantity exceeds four kilograms, punishment
1
[shall be] life imprisonment and fine which may not be less than two and half million.
(3) Whoever contravenes the provisions of sections 6, 7 and 8 regarding controlled
substances specified in Table-I and Table-II of the Schedule-II shall be punishable with punishment
1
Subs. by Act XXXVIII of 2023, s.2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-220-320.jpg)


![Page 19 of 50
on parole as per relevant laws and rules.
(3) Notwithstanding anything contained in any other law for time being in force, imprisonment
for life under this Act means imprisonment in jail for the period of twenty-five years.]
10. Prohibition on owning, operating premises or machinery for manufacture of narcotic
drugs, etc.___
No one shall own, manage, operate or control any premises, place, equipment or
machinery for the purpose of manufacture or production of cannabis, cocaine, opium, opium derivatives,
narcotic drugs, psychotropic substance or controlled substance save in accordance with the conditions of
a licence and payment of such fees as may be prescribed.
11. Punishment for contravention of Section 10.__
Whoever contravenes the provisions of
section 10 shall be punishable with imprisonment which may extend to twenty-five years but shall not be
less than ten years and shall also be liable to fine which shall not be less than one million rupees.
12. Prohibition of acquisition and possession of assets derived from narcotic offences.__
No one shall knowingly___
(a) possess, acquire, use, convert, assign or transfer any assets which have been
derived, generated or obtained, directly or indirectly, either in his own name or in
the name of his associates, relatives or any other person through an act or
omission relating to narcotic substances which constitutes an offence punishable
under this Act, the Customs Act, 1969 (IV of 1969), the Prohibition
(Enforcement of Hadd) Order, 1979 (P.O. No. 4 of 1979), under any other law for
the time being in force, or constituted an offence under any law repealed by this
Act, the Control of Narcotic Substances Ordinance, 1996 (XCIV of 1996) or any
other law repealed by this Act;
(b) hold or possess on behalf of any other person any assets referred to in clause (a);
and
(c) conceal or disguise the true nature, source, location, disposition, movement, title,
or ownership of such assets by making false declaration 1
[or by employing any
other means of concealment] in relation thereto.
13. Punishment for contravention of section 12.__
Whoever contravenes the provisions of
section 12 shall be punishable with imprisonment which may extend to fourteen years but shall notbe
less than five years and shall also be liable to fine which shall not be less than the prevailing valueof the
assets and such assets shall also be liable to forfeiture to the Federal Government.
14. Prohibition on aiding, abetment or association in narcotic offences.__ No one shall,
within or outside Pakistan, participate in, associate or conspire to commit, attempt to commit, aid, abet,
facilitate, incite induce or counsel the commission of an offence punishable under this Act.
Explanation. For the purpose of this section, a person shall be deemed to have associated
with, conspired, aided, abetted, facilitated, incited, induced or counselled an offence within the meaning
of this section if he does anything in a place beyond Pakistan which__
(a) would constitute an offence if committed within Pakistan; or
(b) under the laws of such other place, is an offence relating to narcotic drugs,
psychotropic substances or controlled substances having all the legal oranalogous
conditions required to constitute it as an offence punishable under this Act.
1
Ins. by the control of narcotic substances (Amdt) Act 2020 (XXIV of 2020), s. 2](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-223-320.jpg)
![Page 20 of 50
15. Punishment for contravention of section 14.__ Whoever participates in, associates,
conspires to commit, attempts to commit, aids, abets, facilitates, incites, induces or counsels
the commission of an offence in contravention of section 14 shall, whether such offence be or be not
committed in consequence of such participation, association, conspiracy, aid, abetment, facilitation,
incitement, inducement or counselling, and notwithstanding anything contained in section 116 of the
Pakistan Penal Code (Act XLV of 1860), be punishable with the punishment provided for the
offence or such lesser punishment as may be awarded by the Court.
16. Punishment for offence for which no punishment is provided. __ Whoever
contravenes any provision of this Act or any rule or order made, or any licence, permit or
authorisation issued hereunder, for which no punishment is separately provided in this Chapter, shall
be punishable with imprisonment for a term which may extend to 1
[three years and fine].
17. Obstructions to officers.___ Whoever hinders or obstructs any officer in the
performanceof his functions under this Act or willfully furnishes to such officer any information
which is, to his knowledge or belief, false in material particulars shall be punishable with rigorous
imprisonment for a term which may extend to three years, 1
[and fine but shall not be less than one
year and fine].
18. Limit of fine, etc.__ Where for any offence under this Act no amount of minimum fine
has been fixed, the Special Court shall impose a fine keeping in view the quality and quantity of the
narcotic drug, psychotropic substance or controlled substance involved in the commission of such
offence.
19. Forfeiture of assets of an offender.__ Notwithstanding anything contained in section
13, where the Special Court finds a person guilty of an offence punishable under this Act and
sentences him to imprisonment for 1
[one year or above], the Court shall order that his assets
derivable from trafficking in narcotic substances shall stand forfeited to the Federal Government
unless it is satisfied, for which the burden of proof shall rest on the accused, that they or any part
thereof, have not been so acquired.
_______________
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) ss.7-9.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-224-320.jpg)

![Page 22 of 50
(b) detain and search any person whom he has reason to believe to have
committed an offence punishable under this Act, and if such person has any
narcotic drug, psychotropic substance or controlled substance in his
possession and such possession appears to him to be unlawful, arrest him.
Explanation.__ For the purpose of this section, the expression “public place” includes
any public conveyance, hotel, shop or any other place intended for use by, or accessible to, the
public.
23. Power to stop and search conveyance.__ An officer referred to in Section 1
[21], may,
if he has reason to suspect that any conveyance is, or is about to be, used for the transport of any
narcotic drug, psychotropic substance or controlled substance in respect of which he suspects that
any provision of this Act has been, or is being, or is about to be, contravened at any time, stop such
conveyance or, in the case of an aircraft, compel it to land and—
(a) rummage and search the conveyance or part thereof;
(b) examine and search any goods on or in the conveyance; or
(c) if it becomes necessary to stop the conveyance, he may use all reasonable
force for stopping it.
24. Undercover and controlled delivery operations.__ (1) Subject to sub-section (2) and
to any treaty, arrangement or understanding with any foreign State to which Pakistan may from
time to time be party, the Federal Government may give approval in writing to controlled delivery
operations, for the purpose of gathering evidence in Pakistan or elsewhere relating to the
commission of any offence against this Act or a similar law of a foreign State.
(2) Approval may not be given under sub-section (1) unless the Federal
Government__
(a) suspects that persons, whose identity may or may not be known, have
engaged in, are engaging in or are about to engage in, conduct constituting
an offence against this Act or a similar law of a foreign State; and
(b) is satisfied that the proposed operations are properly designed to give such
persons an opportunity to manifest that conduct or provide other evidence
of it.
(3) The Federal Government may give approval from time to time for a period not
exceeding three months.
(4) Without limiting the generality of sub-section (1), the activities which may be
undertaken by an authorised participant in the course of and for the purposes of a controlled
delivery and undercover operation include__
(a) allowing any conveyance to enter or leave Pakistan;
(b) allowing any narcotic drug, psychotropic substance, manufactured drug,
controlled substance, property or other thing in or on the conveyance to be
delivered or collected;
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.10.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-226-320.jpg)

![Page 24 of 50
28. Powers to invest officers of law enforcement agencies with powers of an officer-in-
charge of a police station.__ The Federal Government may invest any officer of law enforcement
agency or any other officer within their respective jurisdiction with the powers of an officer-in-
charge of a police station for the investigation of offence under this Act.
29. Presumption from possession of illicit articles.__ In trials under this Act, it may be
presumed, unless and until the contrary is proved, that the accused has committed an offence under
this Act in respect of___
(a) any narcotic drug, psychotropic substance or controlled substance;
(b) any cannabis, coca or opium poppy plant growing on any land which he has
cultivated;
(c) any apparatus specially designed or any group of utensils specially adapted for
the production or manufacture of any narcotic drug, psychotropic substance or
controlled substance; or
(d) any materials which have undergone any process towards the production or
manufacture of narcotic drug, psychotropic substance or controlled substance
or any residue left of the materials from which a narcotic drug, psychotropic
substance or controlled substance has been produced or manufactured, for the
possession of which he fails to account satisfactorily.
30. Presumption as to documents in certain cases.__ Where in the course of an
investigation for an offence committed under this Act or any other law for the time being in force
any document is produced or furnished, or has been seized from the custody or control of any
person, the Special Court shall—
(a) presume, unless the contrary is proved, that the signature and every other part
of such document which purports to be in the hand-writing of any
particular person or which the Special Court may reasonably assume to have
been signed by, or to be in the hand-writing of, any particular person, is
in that person’s hand-writing, and in the case of a document executed or
attested, that it was executed or attested by the person by whom it purports to
have been so executed or attested;
(b) admit the document in evidence, notwithstanding that it is not duly stamped, if
such document is otherwise admissible in evidence; and
(c) presume, unless the contrary is proved, the truth of the contents of such
document.
31. Power to call for information.__ (1) An officer authorised under section 21 may, during
the course of an enquiry 1
[or investigation] in connection with the contravention of any
provision of this Act,__
1
Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.11.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-228-320.jpg)
![Page 25 of 50
(a) call for information from any person for the purpose of satisfying himself
whether there has been any contravention of the provisions of this Act or any
rule or order made thereunder;
(b) require any person to produce or deliver any document or thing useful or
relevant to the inquiry ;
(c) examine any person acquainted with the facts and circumstances of the case;
and
(d) require any bank or financial institution, notwithstanding anything contained
in any other law for the time being in force, to provide any information
whatsoever.
1
[(2) Notwithstanding anything contained in any provision of the Income Tax Ordinance
2001 (XLI of 2001), the Sales Tax Act, 1990, the Federal Excise Act, 2005 or any other law for the
time being in force relating to information, submitted by any person with respect to tax purposes, no
government department or authority shall refuse to provide documents and information called by the
officer authorized under this Act.]
32. Articles connected with narcotics.__ (1) Whenever an offence has been committed
which is punishable under this Act, the narcotic drug, psychotropic substance or controlled
substance, materials, apparatus and utensils in respect of which, or by means of which, such
offence has been committed shall be liable to confiscation.
(2) Any narcotic drug, psychotropic substance or controlled substance lawfully imported,
transported, manufactured, possessed, or sold along with, or in addition to, any narcotic drug,
psychotropic substance or controlled substance which is liable to confiscation under sub-section (1)
and the receptacles or packages, and the vehicles, vessels and other conveyances used in carrying
such drugs and substances shall likewise be liable to confiscation:
Provided that no vehicle, vessel or other conveyance shall be liable to confiscation
unless it is proved that the owner thereof knew that the offence was being, or was to be, committed
2
[:]
2
[Provided further that if any currency whether local or foreign or any valuable item having
monetary value used for the commission of the offence under this Act is seized it shall be
confiscated along-with other articles.]
33. Procedure for making confiscation.__(1) In the trial of offences under this Act,
whether the accused is convicted or acquitted, the Special Court shall decide whether any
article frozen or seized in connection with such offence is liable to confiscation.
(2) Where any article seized under this Act appears to be liable to confiscation under
section 32, but the person who committed the offence in connection therewith is not known or
cannot be found, the Special Court may inquire into and decide such liability, and may order
confiscation accordingly:
Provided that no order of confiscation of an article shall be made until the expiry of one
month from the date of freezing or seizure, or without hearing any person who may claim any right
thereto and the evidence, if any, which he produces in respect of his claim:
1
Added by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.11.
2
Subs. and added by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.12.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-229-320.jpg)
![Page 26 of 50
1
[Provided further that if any such article other than a narcotic drug, psychotropic substance
or controlled substance is liable to speedy and natural decay or in case of a vehicle, the Director
General, Anti-Narcotic Force, or any other officer authorized by notification in the official Gazette
by the Federal Government, is of opinion that the sale of such article or vehicle is for the benefit of
its owner, he may, with the approval of the Special Court, after due notice to the owner and by
public auction, direct that the article or, as the case may be, the vehicle be sold in accordance with
the rules made under this Act and the sale proceeds shall be deposited in the National Fund for
Drug Abuse till the final judgment of the court.]
1
[(3) If on adjudication or, as the case may be, in case of appeal the vehicle or, as the case
may be, an article so sold is found not to have been liable to such confiscation, the entire sale
proceeds shall be handed over to the owner.]
(4) A narcotic drug, psychotropic substance or controlled substance seized under this Act
shall be disposed of under Section 516A of the Code of Criminal Procedure, 1898 (Act V of 1898)
2
[:]
2
[Provided that the Federal Government may exempt any narcotic drugs, psychotropic
substance and controlled substance for disposal under section 516A of the Code by making rules
under this Act.]
34. Federal Narcotics Testing Laboratory, etc.__ (1) The Federal Government may, as
soon as may be after the commencement of this Act, set-up a Federal Narcotic Testing Laboratory
and such other institutes and narcotics testing research laboratories or notify any other laboratory or
institute to be a Federal Narcotics Testing Laboratory for carrying out the purposes of this Act.
(2) The Provincial Government may, wherever deems appropriate, set-up Provincial
Narcotics Testing Laboratories.
35. Government Analyst.__ The Federal Government or a Provincial Government may, by
notification in the official Gazette, appoint such persons as it thinks fit, having the prescribed
qualifications, to be Federal Government Analysts or, as the case may be, Provincial Government
Analysts, for such areas and in respect of such narcotic drugs, psychotropic substances or
controlled substances as may be specified in the notification.
36. Reports of Government Analysts.___(1) The Government Analyst to whom a sample
of any narcotic drugs, psychotropic substance or controlled substance has been submitted for test
and analysis shall deliver to the person submitting it, a signed report in quadruplicate in the
prescribed form and forward one copy thereof to such authority as may be prescribed.
(2) Notwithstanding anything contained in any other law for the time being in force, any
document purporting to be a report signed by a Government Analyst shall be admissible as
evidence of the facts stated therein without formal proof and such evidence shall, unless rebutted,
be conclusive.
1
Subs. by Ord. 66 of 2000, s.2.
2
Subs. & added by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.13.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-230-320.jpg)
![Page 27 of 50
CHAPTER IV
FREEZING AND FORFEITURE OF ASSETS
37. Freezing of assets, etc.___(1) Where the Special Court trying an offence punishable
under this Act is satisfied that there appear reasonable grounds for believing that the accused has
committed such an offence, it may order the freezing of the assets of the accused, his relatives and
associates.
(2) Where in the opinion of the Director-General or an officer authorised under section 21
an offence is being or has been committed, he may freeze the assets of such accused and within
1
[thirty] days of the freezing shall place before the Court the material on basis of which the freezing
was made and further continuation of the freezing or otherwise shall be decided by the Court.
(3) The said officer shall trace, identify and freeze the assets during the investigation or trial
for the purpose of forfeiture by the Special Court:
Provided that the Director-General, or as the case may be, the officer freezing any asset
shall, within three days, inform the Special Court about such freezing and the Special Court shall,
after notice to the person whose assets have been frozen, by an order in writing, confirm, rescind or
vary such freezing.
38. Tracing of assets.__(1) On receipt of a complaint or credible information or where a
reasonable suspicion exists about any person that he has acquired assets through illicit involvement
in narcotics, within or without Pakistan, an officer empowered under section 21 or any other person
authorised under section 37, shall proceed to trace and identify such assets.
(2) On receipt of authenticated information from a foreign court of competent jurisdiction
that a citizen of Pakistan has been charged for an offence which is also an offence under this Act,
an officer empowered under section 21 or any other person authorised under section 37 shall
proceed to trace and identify the assets of the said person, and subject to the provision of sub-
section (3) may freeze the said assets.
(3) Information about such assets shall be laid before to the Special Court for the purpose of
section 13 and section 19 in case action under this Act or any other law for the time being in force
is initiated and in case of the person who has committed the offence is outside Pakistan, before the
High Court for the purpose of forfeiture of assets under section 40.
(4) The actions referred to in sub-sections (1) and (2) may include any inquiry,
investigation or survey in respect of any person, premises, place, property, conveyance, documents
and books of accounts.
39. Order for forfeiture of assets.__ (1) Where the Special Court convicts an accused
under section 13, or sentences him to imprisonment for 1
[one year or more], the Director-General
or an officer authorised by him may request the said Court by an application in writing alongwith a
list of the assets of the convict or, as the case may be, his associates, relatives or any other person
holding or possessing such assets on his behalf, for forfeiture thereof.
(2) Where the Special Court is satisfied that any assets specified in the list referred
to in sub-section (1) were derived, generated or obtained in contravention of section 12 or are
liable to be forfeited under section 19, it may order that such assets shall stand forfeited to the
Federal Government 1
[and shall vest in that Government free from all encumbrances]:
1
Subs. and added by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) ss.14-15.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-231-320.jpg)

![Page 29 of 50
1[40A. Jurisdiction of special court. __ No court other than the Special Court established
under this Act shall have the power to entertain any suit or claim relating to the property, which is
pending for adjudication before the Special Court for the purpose of forfeiture of assets under
section 39 or section 40.]
41. Prohibition of alienation of freezed property.__ (1) Where any order under section 37
or section 40 is made for freezing of any asset, any alienation or transfer of such asset by any
manneror mode shall, till the conclusion of the proceedings under this Act, or until such order is
vacated be void, and if such asset is subsequently forfeited to the Federal Government, any such
alienation or transfer of assets shall be deemed to be of no effect whatsoever.
(2) Any person who knowingly alienates or transfers any assets in respect whereof an order
has been made under section 37 or section 40 shall be guilty of an offence punishable, on
conviction,with imprisonment for a term which may extend to three years, or with fine, or with
both.
42. Punishment for acquiring property in relation to which proceedings have been
taken under this Act.__ Any person who knowingly acquires any assets which have been frozen
under this Act shall be punishable with imprisonment for a term which may extend to three years
and with fine.
43. Power to take possession.__ (1) Where any asset is ordered to be forfeited to the Federal
Government under section 39, the Special Court may direct the person holding or possessing
such asset to surrender or deliver its possession to the Administrator appointed under section
44 or any other person authorised by the Special Court in this behalf, within such time as may be
directed by it.
(2) If any person refuses or fails to comply with a direction issued under sub-section
(1), the Special Court may require the Superintendent of Police of the district where such assets are
located to provide police assistance to the Administrator for securing possession thereof, and it
shall be the duty of the Superintendent of Police to comply with such requisition.
44. Management of assets frozen or forfeited under the Act. __ (1) The Federal
Government may, by a notification in the official Gazette, appoint any officer of the Federal
Government or a Provincial Government as it may think fit to perform the functions of an
Administrator of the assets frozen or forfeited under this order.
(2) The Administrator appointed under sub-section (1) shall take such actions and exercise
such powers as the Federal Government may direct for the maintenance and disposal of the assets
which are frozen or forfeited to the Federal Government.
___________
CHAPTER V
SPECIAL COURTS
45. Jurisdiction to try offences.__ The Special Court established under this Act shall have
the exclusive jurisdiction to try an offence cognizable under this Act 1
[and when trying an offence
under this Act, a Special Court may also try in offence other than an offence under this Act when
so authorized by Federal Government in this regard and with which the accused may, under the
Code of Criminal Procedure, 1898 (Act V of 1898), be charged jointly at the same trial.]
1
Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) ss. 16-17.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-233-320.jpg)

![Page 31 of 50
(2) On the establishment of Special Courts under sections 45 and 46, all cases where the
sentence of an offence is two years or less, shall stand transferred to the respective Special Court
comprising a Judicial Magistrate of the First Class and all other cases to the respective
Special Courts comprising of Sessions Judges or Additional Sessions Judges.
(3) Notwithstanding anything hereinbefore contained, a remand may be granted by the
nearest 1
[* * * *] Judicial Magistrate of the First Class.
2[49A. Remand.__ Notwithstanding anything contained in the Code of Criminal Procedure,
1898 (Act V of 1898) or any law for the time being in force, the person arrested under this Act shall
having regard to the facts and circumstances of the case be liable to be detained in custody for the
purpose of inquiry and investigation for a period not exceeding ninety days and court may remand an
accused person to custody not exceeding fifteen days at a time and for every subsequent remand, the
court shall record reasons in writing.]
50. Special Prosecutor.__ (1) The Federal Government may appoint a person who is an
advocate of a High Court to be a Special Prosecutor on such terms and conditions as may be
determined by it and any person so appointed shall be competent to conduct proceedings under this
Act before a Special Court and 2
[any appellate court], if so directed by the Federal Government, to
withdraw such proceedings.
(2) When a Special Prosecutor appointed under sub-section (1) is, for any reason,
temporarily unable to conduct proceedings before the Special Court, the proceedings shall be
conducted by such person as may be authorised in this behalf by the Special Court.
51. No bail to be granted in respect of certain offences.__ (1) Notwithstanding anything
contained in sections 496 and 497 of the Criminal Procedure Code, 1898 (V of 1898) bail shall not
be granted to an accused person charged with an offence under this Act or under any other law
relating to narcotics where the offence is punishable with death.
(2) In the case of other offences punishable under this Act, bail shall not be normally
granted unless the Court is of the opinion that it is a fit case for the grant of bail and against the
security of a substantial amount.
____________
CHAPTER VI
TREATMENT AND REHABILITATION OF ADDICTS
52. Registration of addicts.__ (1) Each Provincial Government shall register all addicts
within their respective jurisdiction for the purpose of treatment and rehabilitation of addicts.
(2) The Federal Government shall bear all expenses for first time compulsory de-
toxification or de-addiction of an addict.
(3) The addict shall carry a registration card in such form as may be prescribed and
produce it to any public authority on demand.
53. Powers of the Government to establish centres for treatment of addicts. __
The
Provincial Government shall establish as many centres as may be deemed necessary for de-
toxification, de-addiction, education, after-care, rehabilitation, social integration of addicts and for
supply of such medicines as are considered necessary for the de-toxification of the addicts.
1
Omitted by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.18.
2
Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) ss.19-20.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-235-320.jpg)


![Page 34 of 50
(2) Such requests shall__
(a) give the name of the authority concerned with the criminal matter to which
the request relates;
(b) give a description of a nature of the criminal matter and a statement
setting out a summary of the relevant facts and laws;
(c) give a description of the purpose of the request and of the nature of the
assistance being sought;
(d) in the case of a request to freeze or forfeit assets believed on reasonable
grounds to be located in Pakistan, give details of the offence, particulars of
any investigation or proceedings commenced in respect of the offence, and
be accompanied by a copy of any relevant freezing or forfeiture order of the
Court;
(e) give details of any procedure that the foreign State wishes to be followed by
Pakistan in giving effect to the request, particularly in the case of a request to
take evidence;
(f) contain a statement setting out any wishes of the foreign State concerning
any confidentiality relating to the request and the reasons for those wishes;
(g) give details of the period within which the foreign State wishes the request
to be complied with;
(h) state, where applicable, the grounds for believing that the relevant assets or
things to be traced, frozen or seized are located in Pakistan; and
(i) contain any other information that may assist in giving effect to the request.
(3) A request may be accepted, after consultation, notwithstanding that the request, as
originally made, does not comply with sub-section (2).
59. Foreign requests for an evidence-gathering Order or a search warrant.__ (1)
Notwithstanding anything contained in any law for the time being in force, where the Federal
Government approves a request of a foreign State pursuant to section 1
[58] to obtain evidence in
Pakistan, or be able to be given by a person believed to be in Pakistan, the Director-General or an
officer authorised by him may apply to the High Court for__
(a) a search warrant; or
(b) an evidence-gathering order.
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.21.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-238-320.jpg)



![Page 38 of 50
61. Foreign persons in Pakistan in response to a Pakistan request.___(1) The Federal
Government may, by written notice, authorize the temporary detention in Pakistan of a person in
detention in a foreign State who is transferred from that State to Pakistan pursuant to a request
under clause (f) of (1) of Section 1
[57], for such period as may be agreed with that State for the
purposes of the request, and the return in custody of the person to the foreign State.
(2) A person in respect of whom a notice is issued under sub-section (1) shall, so long as the
notice is in force__
(a) be permitted to enter Pakistan and remain in Pakistan for the purposes of
the request, and to leave Pakistan when no longer required for those
purposes, notwithstanding any Pakistan law to the contrary; and
(b) while in custody in Pakistan for the purposes of the request, be deemed to be
in lawful custody.
(3) The Federal Government may at any time vary a notice under sub-section (1), and
where the foreign State requests the release of the person from custody, either immediately or on a
specified date, the Federal Government shall direct that the person be released from custody
accordingly.
(4) The provisions of this section shall apply, mutates mutandis, in the case of any detained
person in transit through Pakistan from one foreign State to another pursuant to a request for
assistance of the kind referred to in this section.
(5) Any person, whether or not a detained person, who is in Pakistan in response to a
Pakistan request under this Chapter to give evidence in a proceeding or to give assistance in
relation to an investigation, prosecution or the related proceeding may not, while in Pakistan, be__
(a) detained, prosecuted or punished; or
(b) subjected to civil process, before the person’s departure from the foreign
State pursuant to the request in respect of any act or omission that occurred.
62. Foreign requests for Pakistan restraining orders.__(1) Notwithstanding anything
contained in any law for the time being in force, where the Federal Government approves a request
of a foreign State pursuant to 1
[sub-section (1) of section 63] to restrain dealings in any assets,
some or all of which are believed on reasonable grounds to be located in Pakistan, the Federal
Government may apply to the High Court for a restraining order.
(2) The High Court, to which an application is made under sub-section (1), may issue
a freezing order, where it is satisfied that there are reasonable grounds to believe that__
(a) an offence has been committed, or is suspected on reasonable grounds to
have been committed by a person against the laws of the requesting State
which, if committed in Pakistan, would have constituted an offence under
this Act ;
(b) an investigation or proceeding has commenced in the foreign State relating
to that offence ;
1
Subs. by the control of narcotic substances (Amdt.) A0ct 2022 (XX of 2022) ss.22-23.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-242-320.jpg)
![Page 39 of 50
(c) assets derived by the person, his relatives and associates from the
commission of the offence are located in Pakistan ; and
(d) an order has been made, or is likely to be made in the foreign country
having, to the effect of forfeiting such assets, this 1
[Act] shall apply as if the
offence had been committed in Pakistan, whereupon the freezing order had
been made under sub-section (2) of section 38.
63. Requests for enforcement of foreign confiscation or restraining orders.__
(1) This
section does not apply to cases falling within section 40 of this Act.
(2) Where a foreign State requests the Federal Government to make arrangements for the
enforcement of a__
(a) foreign restraining order; or
(b) foreign forfeiture order,
the Director-General may apply to the High Court for registration of the orders
issued by a Court of that State.
(3) The High Court shall, on application by the Director-General, register the foreign
restraining order if the Court is satisfied that the order is in force in the foreign State.
(4) The High Court shall, on application by the Director-General or an officer authorised by
him, register the foreign forfeiture order if the Court is satisfied__
(a) the order is in force in the foreign State and is not subject to appeal; and
(b) where the person, the subject of the order, did not appear in the foreign
forfeiture order proceedings in the foreign State, that__
(i) the person was given notice of the proceedings in sufficient time to
enable him or her to defend him; or
(ii) the person died or absconded before such notice could be given.
(5) Where a foreign restraining order or foreign forfeiture order is registered in accordance
with this section, a copy of any amendments made in the order in the foreign State (whether before
or after registration), may be registered in the same way as the order, but shall not have effect for
the purposes of this Act until they are so registered.
(6) The High Court shall, on application by the Director-General or an officer authorised by
him, cancel the registration of__
(a) a foreign restraining order if it appears to the Court that the order has ceased
to have effect; and
(b) a foreign forfeiture order if it appears to the Court that the order has been
satisfied or has ceased to have effect.
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.23.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-243-320.jpg)

![Page 41 of 50
CHAPTER IX
GENERAL
67. Reporting of suspicious financial transactions. __ (1) Notwithstanding anything
contained in any law for the time being in force, all banks and financial institutions shall pay
special attention to all unusual patterns of transactions, which have no apparent economic or lawful
purpose and upon suspicion that such transactions could constitute or be related to illicit
narcotics activities, the manager or director of such financial institution shall report the suspicious
transactions to the Director-General.
(2) Whoever fails to supply the information in accordance with sub-section (1) shall be
punishable with rigorous imprisonment which may extend to three years, or with fine, or with both.
68. Presumption to the assets acquired through dealing in narcotics.__ Where there is
reasonable ground to believe that the assets of a person or any part thereof were acquired before or
at the time of, or after the commission of an offence under this Act and there was no other likely
source of acquiring such assets or part thereof, it shall be presumed, unless the contrary is proved,
that such assets or part thereof were acquired, generated or obtained through cultivation,
manufacture, production, sale, purchase, dealing or trafficking of narcotic drugs, psychotropic
substances or controlled substances.
69. Departments to render assistance to the Special Courts, etc.__ All departments of the
Government, banks, financial institutions, corporate bodies, companies, societies and agencies
shall assist the Special Court, Director-General or any other officer authorised by the Federal
Government for the purposes of any inquiry, tracing of any assets or for ascertaining whether any
assets held by any person, his associate or relative were acquired by committing any offence under
this Act.
70. Notice or order not to be invalid for error in description.__ No notice issued, or
order passed, under this Act shall be invalid by reason of any error in the description of the person
or assets specified therein if such person or assets are otherwise identifiable from the description
specified in such notice or order.
71. Delegation.__ (1) The Federal Government may, by notification in the official Gazette
and subject to such conditions and limitations as may be specified in the notification, delegate all
or anyof its powers and functions under this Act as it may deem necessary or expedient to the
Provincial Government, Director-General or any other authority or officer of the Federal
Government.
(2) The Provincial Government may, by notification in the official Gazette, subject to
such conditions and limitations as may be specified in the notification, delegate all or any of its
powers and functions under this Act as it may deem necessary or expedient, to any authority or
officer of that Government.
72. Application of the Customs Act, 1969.__ All prohibitions and restrictions imposed by
or under this 1
[Act] on the import into, export from, Pakistan and transshipment of narcotic
drugs, psychotropic substances or controlled substances shall be deemed to be prohibitions and
restrictions imposed by or under the Customs Act, 1969 (IV of 1969), and the provisions of this
Act shall apply accordingly:
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.24.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-245-320.jpg)
![Page 42 of 50
Provided that, notwithstanding anything contained in the Customs Act, 1969 (IV of 1969),
or any other law for the time being in force, all offences relating to narcotic drugs, psychotropic
substances or controlled substances shall be tried under the provisions of this Act :
Provided further that where the Officers of Customs apprehends a person involved in
any offence relating to narcotic drugs, psychotropic substances or controlled substances shall be
empowered to carry out inquiry and investigation in the same manner as an officer authorised
under this Act.
73. Saving of Provincial and special laws.__ (1) Nothing contained in this Act or in the
rules made thereunder shall affect the validity of any Federal or Provincial law for the time being in
force, or of any rule made thereunder which imposes any restriction or provides for a punishment
not imposed by or provided for under this Act or imposes a restriction or provides for a punishment
greater in degree than a corresponding restriction imposed by or a corresponding punishment
provided for by or under this Act for the cultivation of cannabis plant or consumption of, or traffic
in, any narcotic drug or psychotropic substance within Pakistan or other similar matters.
74. Application of other laws.__ If an offence punishable under this Act, is also an offence
in any other law for the time being in force, nothing in that law shall prevent the offender from
being punished under this Act :
Provided that nothing contained in section 523 of the Code of Criminal Procedure, 1898
(Act V of 1898), or any other provision of the said Code or any other law for time being in force,
the custody of narcotic drugs, psychotropic substances, controlled substances, any material utensils
used for production or manufacture of such drugs or substances or any conveyance used in import,
export, transport or transhipment thereof or for commission of an offence under this Act, shall not
be given on custody to the accused or any of his associate or relative or any private individual till
the conclusion of the case 1
[except as provided in the second proviso to sub-section (2) of section
33].
2[74A.Power to amend Schedules.__ The Federal Government may, by notification in the
official Gazette, amend the Schedules so as to add any entry thereto, amend any entry therein or
omit any entry there from if it is satisfied that it is necessary or expedient so to do on the basis of
following, namely:__
(a) the information and evidence which has become available to it with respect to
the nature and effects of and the abuse or the scope for abuse of any substance
(natural or synthetic) or natural material or preparation of such substance or
material; or
(b) the modifications or provisions, if any, which have been made to or in any
international convention with respect to such substance, natural material or any
salt or preparation of such substances or material.]
75. Indemnity.__ No suit, prosecution or other proceedings shall lie against the Federal
Government or Provincial Government or any officer of the Federal Government or of a Provincial
Government for anything in good faith, done or intended to be done in pursuance of this Act or the
rules made thereunder.
76. Act to override other laws.__ The provisions of this Act shall have effect
notwithstanding anything contained in any other law for the time being in force.
1
Added by Ord. 66 of 2000, s.3.
2
Ins. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.25.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-246-320.jpg)

![Page 44 of 50
1
[SCHEDULE - I]
[See Section 2(za)]
PSYCHOTROPIC SUBSTANCES
_______________________________________________________________________________________________________________
International
non-proprietary
names
Other
non-proprietary
or trivial names
Chemical Name
1 2 3
1. AMFETAMINE Amphetamine (+/-)-a-methylpenethylamine.
2. AMOBARBITAL 5-ethyl-5-barbituric acid.
3. ALLOBARBITAL 5,5-deallylbarturic acid.
4. ALPRAZOLAM 8-chloro-l-methyl-6-phenyl-4H-s-triazoIo
[4, 3-a] [1,4] benzodiazepine.
5. AMFPRAMONE 2-(diethyiamino) propiolphenone.
6. BROLAMFETAM1NE DOB (+/-)4-bromo-2, 5-dimethoxy-a-
methylphenethylamine.
7. BUPRENORPHINE
21-cyclopropyl-7-a-[(s)-1-hydroxy-1,
2,2-trimethylporpyI]-6, 14-endo-ethano-6,
7,8,14-tetrahydrooripavine.
8. BUTALB1TAL 5-allyl-5-isobutyIbarbituric acid.
9. BARBITAL 5,5-diethylbarbituric acid.
10. BENZFETAM1NE benzphnetamine N-benzyl-N, a-dimethylphenethylmine.
11. BROMAZEPAM
butobarbital
7-bromo-l, 3-dihydro-5 (2-pyridyl)
2H-1, 4-benzodiazepin-2-one.
5-butyl-5-thylbarbituric acid.
12. CATHINONE
DET
(-)-(S)-2 aminopropiophenone.
3-[2(diethylamino) ethyl] indole.
DMA (+/-)-2,5-dimethoxy-a-methylphenethy lamine.
DMHP 3-(1,2-dimethyheptyle)-7,8,9,10-tetrahy-
dro-6,6,9-trimethyl-6H-dibenzo [b,d] [pyran-1-
01].
13. CATHINE (+)-norpseudo
ephedrine
(+)-(R)-a-[(RO-l-aminoethyI] benzyl
alcohol.
[DMT 3-[2-diethylamino)ethyl] indole.
[DOET (+/-)-4-ethyl-2,5-dimethoxy-a-Phenethly-amine.
1
Subs. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.26.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-248-320.jpg)
![Page 45 of 50
1 2 3
14. CYCLOBARBITAL 5-(I-cychlohexen-l-yl)-5-ethylbarbituric acid.
15. CAMAZEPAM 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-
phenyl-2Hl,4- benzodiazepin-2-one
dimethylcarbamate (ester).
16. CHLORDIAZEPOXIDE 7-chloro-2-methylmino-5-phenyl-3H-1, 4
benzodiazepine-4-oxide.
17. CLOBAZAM 7-chloro-1 (methyl)-5-phenyl-1H-5-
benzodiazepine-2,4 (3H,5H)-dione.
(+/-)-4-ethyl-2,5-dimethoxy-a-Phenethly-
amine.
5-(l-cychlohexen-l-yl)-5-ethylbarbituric acid.
18. CLONAZEPAM 5-(0-chlorophgenyl)-1,3-dihydro-7-nitro-2H-
1,4-benzodiazpine-2-one.
19. CLORAEPATE 7-chloro-2,3-dihydro-2-oxo-5-phenyl-lH-l,4-
benzodiazepine-3- carboxylic acid.
20. CLOTIAZEPAM 5-(o-chlorphenyl)-7-ethyl-1,3-dihydro-1-
methyl-2H-thieno [2,3-e]-1,4-diazepin-2-one.
21. CLOXAZOLAM 10-chloro-llb-(0-chlorphenyl)-2-3,7,11b-
tetraphydrooxazolo [3,2- d][l,4]
benzodiazepin-6(5H)-one.
22. DEXAMPHETA-
MINE
dexamphe (+)-a-methylphenethylamine
23. DELORAZEPAM 7-Chloro-5-(0-chlorophenyl)-1,3-dihydro-2H-
1,4-bezodiazepin-2-one
24. DIAZEPAM 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-
1,4-benzodiazepine-2-one.
25. ESTAZOLAM 8-chloro-6-penyl-4H-S-triazolo-[4,3-a][1,4]
benzodiazepine.
26. ETHCHLORVYNOL 1-chloro-3-ethyl-1-penten-4yn-3-01.
27. ETHYL LOFLAZEPATE ethyl 7-chloro-5-(0-flurophenyl)-2,3-dihydro-
2-oxo-1H, 4-benzodiaze-pine-3-carboxylate.
28. ETILAMFETA M1NE N-ethylampetamine N-ethyl-a-methylphenethylamine.
29. ETHINAMATE 1-eithynycychlohexanol-carbamate.
30. ETICYCLIDINE PCE N-ethyl-1-phenylcychlohexylamine.
31. FENETYLLINE 7-[2-[a-methlyphenthyl) amino]ethyle]
theophylline.
32. FENCAMFAMIN N-ethyl-3-phenyl-2-norbornanamine.
33. FENPROPOREX [+/-)-3-](a-methlyphenthyl)amino]
propionitrile.
34. FLUD1AZEPAM 7-chloro-5-(0-fluorophenyl)-1,3-dihydro-
1-methyl-2H-1, 4- benzodiazepin-2-one.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-249-320.jpg)
![Page 46 of 50
1 2 3
35. FLUNITRAZEPAM 5-(0-fluorophenyl)-1,3-dihydro-1-methyl-7-
nitro-2H-1,4- benzodiazepin-2-one.
36. FLURAZEPAM 7-chloro-1-[2-diethylamino)ethyl]5-(0-
fluorophenyl)-1,3-dihydro-2H-1,4-
benzodiazepine-2-one.
37. GLUTETHIM1DE 2-ethyl-2-phenylglutarimide 4-benzodiazepin-
2-one.
38. HALAZEPAM 7-chloro-1, 3-dihydro-5-phenyl-1(2,2,2-
trifluororethyyl)-2H-1,4-benzodiazepin-2-one.
39. HALOXAZOLAM 10-bromo-11 b(0-fluorophenyl)-2, 3, 7, 11 b-
tetrahydrooxazolo [3,2-d] [1.4]
benzodiazepine-6 (5H)-one.
40. KETAZOLAM 11-chloro-8, 12b-dihydro-2,8-dimethyl-12b-
phenyl-4H-[l,3] oxazino [3,2,-d][1,4]
benzodiazepine-4,7(6H)-clione.
41. (+)-LYSERGIDE LSD,
LSD-25
9, 10-didehydro-N, diethyl-6
methylergoline-8B-Carboxamide.
MDMA (+/-) N, a-demethyl-3, 4-(methylinendioxy)
phenethylamine.
Mescaline 3,4,5-trimethoxyphenethylamine.
4-methylaminorex (+ /-)-cis-2-amno-4-methyl-5-phenyl-2-
oxazoline.
MMDA 2-methoxy-a-methyl-4,5-(methylenedioxy)
phyenthylamine.
N-ethyl
MDA
(+ /-)-N-ethyl-a- methyl-3, 4-
methylendedioxy) phenethylamine.
N-hydroxy MDA (+ /-)-N-[a-methyl-3,4-(methylendedioxy)
phenethyl] hydroxylamine.
Parahexyl 3-hexyl-7,8,9,10-tetrahydro-6,6,9-trimethyl-
6H- dibenzo [b,d] pyran-1-01.
PMA p-methoxy-a-methylphenethylamine.
psilcine, psilotsin 3-[2-dimethylamino) ethyl]indol-4-01.
42. LEFETAMINE SPA (-)-N,N-dimethyl-l,2-diphenyiethylamine.
43. LOPRAZOLAM 6-(0-chlorophenyl)-2,4-dihydro-2- [(4-methyl-
1-peperazinyl) methylene]-8-nitro-lH-imidazo
[l,2- a][l,4] benzodiazepin-1-one.
44. LOPRAZEPAM 7-chloro-5-(0-chlorophenyl)-l,3-dihydro-3-
hydroxy-2H-1,4-benzodiazepin-2-one.
45. LORMETAZEPAM 7-chloro-5-(0-chlorophenyl)-l,3-dihydro-3-
hydroxy-1-methyl-2H-1,4-benzodiazepine-2-
one.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-250-320.jpg)
![Page 47 of 50
1 2 3
46. LEVAMFETAMINE Levamphe-
Tamine
levomethamphetamine
(-)(R)-a-methylphetyphenethylamine.
(-)-N,a-dimethylphenethylamine.
47. METAFETAMINE methamphetamine (+)-(S)-N, a-dimethylphethylamine.
48. METAFETAMINE
RACEMATE
metafetamine racemate (+ /-)-N,a-dimethylphethylamine.
49. METHYLPHENIDATE Methyl a-phenyl-2-piperidinoacetate.
50. MEPROBAMATE 2-methyl-2-propyl-l,3-propanediol
dicarbamate.
51. METHAQUALONE 2-methyl-3-0-tolyl-4(3H)-quinazolinone.
52. METHYLPHENOBARBITAL 5-ethyl-l-methyl-5-phenyl-barbituric acid.
53. METHYPRYLON 3,3-diethyl-5-methyl-2,4-piperidine-dione.
54. MAZINDOL 5-(p-chlorophenyl)-2,5-dihydro-3H-
imidazo[2,1-a]isoindol-5-01.
55. MEDAZEPAM 7-chloro-2, 3-dihydro-l-methyl-5-phenyl- IH-
1,4-benzodiazepine.
56. MEFENOREX N-(3-chloroporpyl)-a-methylphenethyla-
mine.
57. MIDAZOLAM** 8-chloro-6-(0-fluorophenyl)-l-methyl-
4H-imidazo [1.5-a][l,4]benzodiazepine.
58. NIMETAZEPAM 1, 3-dihydro-l-methyl-7-nitro-5-phenyl-
2H-1,4-benzodiazepin-2-one.
59. NITRAZEPAM 1,3-dihydro-7-nitro-5-phenyl-2H-1, 4-
benzdiazepin-2-one.
60. NORDAZEPAM 7-chloro-1, 3-dihydro-5-phenyl-2H-1, 4-
benzodiazepin-2-one.
61. OXAZEPAM 7-chloro-1,3-dihydro-5-phenyl-2H-1, 4-
benzodiazepine-2-one.
62. OXAZOLAM 10-chloro-2,3,7,11b-tetrahydro-2-methyl-11b-
phenyloxazolo [3,2-d][1,4] benzodiazepine-6-
(5H)-one.
63. PHENCYCLIDINE PCP 1-(1-phencylcychlohenxyl) piperidine.
64. PENTAZOSINE
(2R, 6R, 11R)-1,2,3,4,5,6-hexahydro-6,
ll-dimethyl-3-(3-methyl-2-butenyl)-2, 6-
methono-3-benzazocin-8-01.
65. PHENMETRAZINE
3-methyl-2-phenylmorpholine.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-251-320.jpg)
![Page 48 of 50
1 2 3
66. PENTOBARBITAL
5-ethyl-5-(l-methylbutyl) barbituric acid.
67. PHENOBARBITAL
5-ethyl-5-phenyl barbituric acid.
68. PIPRADROL a,a-diphenyl-2-piperidinemethanol.
69. PSILOCYBINE 3-(2 dimethylamino) ethyl)-indol-4-yl-
dihydrogen phosphate.
70. PEMOLINE 2-amono-5-phenyl-2-oxazolin-4-one (-2-
imino-5-phenyl-4-oxazolidinone).
71. PHENDIMETRAZINE (+)-(2s,3S)-3,4-dimethyl-2-phenylmorpholine
72. PHENTERMINE a,a-dimethylphenethylamine.
73. PINAZEPAM 7-chloro-l-cyclopropylmethyl)-l, 3- dihydro-5-
phenyl-2H-1,4-benzodiazepin-2-one.
74. PRAZEPAM 7-chloro-1-cyclopropyimethyl)-1, 3-dihydro-
5- phenyl-2H-1,4-benzodia-zepin-2-one
75. PYROVALERONE 4’-methyl-2-(1-pyrrolidinyl) valerophenone.
76. ROLICYCLIDINE PHP, PCPY
STP, DOM
1-(I-phenylcyclohexyl) pyrrolidine 2, 5-
dimethoxy-a,4-dimethylphenethylamine.
77. SECOBARBITAL 5-allyl-5-(l-methylbutyl) barbituric acid.
Delta-9-tetrahydrocannabinol
and its stero-chemical variants.
(6aR, 10aR)-6a, 7,8, 10a-tetrahydro-6,6,9-
trimethyl-3-pentyl-6H-dibenzo [b, d] pyran-1-
01.
78. SECBUTABARBITAL 5-sec-butyl-5-ethylbarbituric acid.
79. TEMAZEPAM 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-
phenyl-2H-1,4-benzodiazepin-2-one.
80. TETRAZEPAM 7-chloro-5-(l-cyclohexen-l-yl)-l,3-dihydro-1-
methyl-2H-1,4-benzodiazepin-2-one.
81. TRIAZOLAM 8-chloro-6(0-chlorophenyl)-l-methyl-4H-s-
trizazolo[4, 3a][l,4] bezodiazepine.
82. TENAMFETAMINE MDA a-methyl-3,4-(methylenedioxy),
phenethylamine.
83. TENOCYCLINDINE TCP l-[l-(2-thienyl) clohexyl) piperidine.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-252-320.jpg)
![Page 49 of 50
1 2 3
tetrahydrocannabinol the following isomers and their stereochemical variants.
7,8,9,10-tetrahydro-6, 6, 9-trimethyl-3-pentyl-
6H-dibenzo [b,d] pyran-1-01.
(9R, 10aR)-8,9,10,10a-tetraphydro-6,6, 9-
trimethyl-3-pentyl-6H-dibenzo [b,d] pyran-1-
01.
(6aR, 9R, l0aR) 6a, 9, l0a-tetrahydro-6,6,9-
trimethyl-3-pentyl-6H-dibenzo [b,d] pyran-1-
01.
(6aR, 9R, l0aR) 6a,7, 10, 10a-tetrahydro-
6,6,9-trimethyl-3-pentyl-6H-dibenzo [b,d]
pyran-1-01.
6a. 7, 8, 9-tetrahydor-6,6,9-trimethyl-3-
pentyl-6H-dibenzo [b,d] pyran-1-01.
6a, 7, 8, 9-tetrahydor-6,6,9-trimethyl-3-
pentyl-6H-dibenzo [b,d] pyran-1-01.
(6a R, I0aR)-6a,7, 8,9, 10, 10a-hexahiydro-6,
6,9- trimethyl-3-pentyl-6H-dibenzo [b,d]
pyran 1-01.
84. VINYBITAL 5-(l-methylbutyl)-vinylbarbituric acid.
85. MECLOQUALONE 3(0-chlorophenyl)-2-methyl-4(3H)-
quinazolione.
______________________
1[SCHEDULE-II
[see section 2(k)]
Division –I
(Table-I of the 1988 Convention)
Ephedrine N-acetylanthranilic acid
Erogometrine Isosafrole
Ergotamine 3,4 methylnedeioxphenyl-
Lysergic acid 2- propanone
4-pheny 1-2 propanone Piperonal
Pseudoephedrine Safrole
1
Added. by the control of narcotic substances (Amdt.) Act 2022 (XX of 2022) s.26.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-253-320.jpg)
![Page 50 of 50
Division-II
(Table-II of the 1988 Convention)
Acetic anhydride Hydrochloric acid
Acetone Methyl ethyl
Anthranilic acid Ketone
Ethyle eter Potassium permanaganate
Phenylacetic acid Sulphuric acid
Vb Piperidine Toluene ]](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-254-320.jpg)

![Page 1 of 4
THE POISONS ACT, 1919
CONTENTS
1. Short title and extent
2. Power of the Provincial Government to regulate possession for sale and sale of any poison
3. Power to prohibit importation into Pakistan of any poison except under licence
4. Power to regulate possession of any poison in certain areas
5. Presumption as to specified poisons
6. Penalty for unlawful importation, etc.
7. Power to issue search warrants
8. Rules
9. Savings
10. [Repeal]](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-256-320.jpg)
![Page 2 of 4
THE POISONS ACT, 1919
2
ACT No. XII OF 1919
[3rd September, 1919]
An Act to consolidate and amend the law regulating the importation, possession and sale of poisons
3
* * *.
WHEREAS it is expedient to consolidate and amend the law regulating the importation,
possession and sale of poisons 4
* * *;
It is hereby enacted as follows:-
1. Short title and extent.___
(1) This Act may be called the Poisons Act, 1919.
5
[(2) It extends to the whole of Pakistan.]
2. Power of the Provincial Government to regulate possession for sale and sale of any
poison.___
(1) 6
* * *, the 7
[Provincial Government] may by rule regulate within the whole or any part
of the territories under its administration the possession for sale and the sale, whether wholesale or
retail, of any specified poison.
(2) In particular, and without prejudice to the generality of the foregoing power, such rules may
provide for–
(a) the grant of licences to possess any specified poison for sale, wholesale or retail,
and the fixing of the fee (if any) to be charged for such licences ;
(b) the classes of persons to whom alone such licences may be granted ;
(c) the classes of persons to whom alone any such poison may be sold ;
(d) the maximum quantity of any such poison which may be sold to any one person;
(e) the maintenance by vendors of any such poison of registers of sales, the
particulars to be entered in such registers, and the inspection of the same ;
(f) the safe custody of such poisons and the labelling of the vessels, packages or
coverings in which any such poison is sold or possessed for sale ; and
(g) the inspection and examination of any such poison when possessed for sale by
any such vendor.
2
For Statement of Objects and Reasons, see Gazette of India, 1919, Pt. V, p. 22 ; and for Proceedings in Council, s., 1919, Pt. VI, pp. 170 and 872.
This Act has been applied to Phulera in the Excluded Area of Upper Tanawal to the extent the Act is applicable in the N.-W.F.P., subject to certain
modifications ; and extended to the Excluded Area of Upper Tanawal (N.- W.F.P.) other than Phulera with effect from such date and subject to such
modifications as may be notified, see N.- W.F.P. (Upper Tanawal) (Excluded Area) Laws Regulation, 1950.
It has also been extended to the Leased Areas of Baluchistan, see the Leased Areas (Laws) Order, 1950 (G. G. O. 3 of 1950) ; and applied in the Federated
Areas of Baluchistan, see Gazette of India, 1937, Pt. 1, p. 1499.
3
The words "throughout British India" omitted by A. O., 1949 Sch.
4
Subs. by the Central Laws (Statute Reform) Ordinance, 1960 (21 of 1960), s. 3 and 2nd Sch. (with effect from the 14th October, 1955), for the original
sub-section (2) as amended by A. O., 1949, and the Federal Laws (Revision and Declaration) Act, 1951 (26 of 1951), s. 8.)
5
The words "Subject to the control of the G. G. in C." rep. by A. O., 1937.
6
Subs. ibid., for "L. G.".](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-257-320.jpg)
![Page 3 of 4
3. Power to prohibit importation into Pakistan of any poison except under licence. The
1
[Federal Government] may, by notification in the 2
[official Gazette], prohibit, except under and in
accordance with the conditions of a licence, the importation into 3
[Pakistan] 4
[across any customs
frontier defined by the 5
[Federal Government]] of any specified poison, and may by rule regulate the
grant of licences.
4. Power to regulate possession of any poison in certain areas.___
(1) The 6
[Provincial
Government] 7
* * * may by rule regulate the possession of any specified poison in any local area in
which the use of such poison for the purpose of committing murder or mischief by poisoning cattle
appears to it to be of such frequent occurrence as to render restrictions on the possession thereof
desirable.
(2) In making any rule under sub-section (1), the 6
[Provincial Government] may direct that any
breach thereof shall be punishable with imprisonment for a term which may extend to one year, or with
fine which may extend to one thousand rupees, or with both, together with confiscation of the poison
in respect of which the breach has been committed, and of the vessels, packages or coverings in which
the same is found.
5. Presumption as to specified poisons. Any substance specified as a poison in a rule made
or notification issued under this Act shall be deemed to be a poison for the purposes of this Act.
6. Penalty for unlawful importation, etc.___
(1) Whoever-
(a) commits a breach of any rule made under section 2, or
(b) imports 8
* * * without a licence 9
[into 3
[Pakistan] across a customs frontier
defined by the 10
[Federal Government]] any poison the importation of which is
for the time being restricted under section 3, or
(c) breaks any condition of a licence for the importation of any poison granted to
him under section 3, shall be punishable,___
(i) on a first conviction, with imprisonment for a term which may extend to
three months, or with fine which may extend to five hundred rupees, or
with both, and
(ii) on a second or subsequent conviction, with imprisonment for a term
which may extend to six months, or with fine which may extend to one
thousand rupees, or with both.
1
Subs. by F.A.O., 1975, Art. 2 and Table, for "Central Government", which was previously amended by A. O., 1937, for "G. G. in C.".
2
Subs. by A.O. 1937 for "Gazette of India".
3
Subs. by the Central Laws (Statute Reform) Ordinance, 1960 (21 of 1960), s. 3 and 2nd Sch. (with effect from the 74th October, 1955), for " the Provinces
and the Capital of the Federation" which had been subs. by A. O., 1949, Arts. 3 (2) and 4, for "British India".
4
Ins. by A. O., 1937.
5
Subs. by F.A.O., 1975 Art. 2 and Table, for "Central Government".
6
Subs. by A. O., 1937 for "L. G.".)
7
The words “subject to the control of the G.G. in C." were rep. by A. O., 1937. The word s"subject to the control" had been substituted for the words
“with the previous sanction" by the Devolution Act, 1920 (38 of 1920), s. 2 and Sch. I.
8
The words "into British India" rep. by. A. O., 1937.
9
Ins., ibid.
10
Subs. by F. A. O., 1975, Art. 2 and Table, for “Central Government”.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-258-320.jpg)
![Page 4 of 4
(2) Any poison in respect of which an offence has been committed under this section, together
with the vessels, packages or coverings in which the same is found, shall be liable to confiscation.
7. Power to issue search warrants.___
(1) The District Magistrate, 1
[and] the Sub-divisional
Magistrate 2
* * *, may issue a warrant for the search of any place in which he has reason to believe or
to suspect that any poison is possessed or sold in contravention of this Act or any rule thereunder, or
that any poison liable to confiscation under this Act is kept or concealed.
(2) The person to whom the warrant is directed may enter and search the place in accordance
therewith, and the provisions of the Code of Criminal Procedure, 1898 (V of 1898), relating to search
of warrants shall as far as may be, be deemed to apply to the execution of the warrant.
8. Rules.-(1) In addition to any other power to make rules hereinbefore conferred 3
* * * the
4
[Provincial Government] may make rules generally to carry out the purposes and objects of this Act
5
[except section 3].
(2) Every power to make rules conferred by this Act shall be subject to the condition of the
rules being made after previous publication.
(3) All rules made by the 6
[Federal Government] or by the 7
[Provincial Government] under this
Act shall be published in the 8
[official Gazette] and on such publication shall have effect as if enacted
in this Act.
9. Savings.___
(1) Nothing in this Act or in any licence granted or rule made thereunder shall
extend to, or interfere with, anything done in good faith in the exercise of his profession as such by a
medical or veterinary practitioner.
(2) Notwithstanding anything hereinbefore contained, the 7
[Provincial Government] may 9
* *
* by general or special order declare that all or any of the provisions of this Act 10
[except section 3]
shall be deemed not to apply to any article or class of articles of commerce specified in such order, or
to any poison or class of poisons used for any purpose so specified.
(3) The authority on which any power to make rules under this Act is conferred may, by general
or special order, either wholly or partially-
(a) exempt from the operation of any such rules, or
(b) exclude from the scope of the exemption provided by sub-section (1),
any person or class of persons either generally or in respect of any poisons specified in
the order.
10. [Repeal of Act I of 1904.] Rep. by the Repealing Act. 1927 (XII of 1927).
Date: 11-09-2024
1
Ins. by A. O., 1949, Sch
2
The words and commas "and, in a Presidency-town, the Commissioner of Police" omitted ibid.
3
The words "and subject to the control of the G. G. in C." were rep. by A. O., 1937. The word "and" had been subs., for the words "the G. G.. in C. or"
by the Devolution Act, 1920 (38 of 1920), s. 2 and Sch. I.
4
Subs. by A.O 1937 for “L.G”
5
Ins. A. O., 1937.
6
Subs. ibid., for "L. G."
7
Subs. by para. 4 of A. O., 1937, for "Gazette of India or the local official Gazette, as the case may be". Strictly the substitution would read "official
Gazette or the official Gazette, as the case may be" but the last nine words have been omitted as being obviously redundant
8
The words "in its discretion" rep. by A. O., 1937
9
Ins. ibid.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-259-320.jpg)




![69 Penalty for failure to display certain notices.
70 Determination of "occupier" for purposes of this Chapter.
71 Exemption of occupier or manager from liability in certain cases.
72 Presumption as to employment.
73 Evidence as to age.
74 Cognizance of offences.
75 Limitation of prosecutions
CHAPTER VII
SUPPLEMENTAL
76 Display of factory notices.
77 Power of Provincial Government to make rules
78 [Repealed.]
79 Publication of rules.
80 Application to Government factories.
81 Protection to persons acting under this Act.
82 [Repealed.]
THE SCHEDULE.
[Repealed.]
Page 4 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-264-320.jpg)
![THE FACTORIES ACT, 1934
1ACT NO.XXV OF 1934
[20th August, 1934]
An Act to consolidate and amend the law regulating labour in factories.
WHEREAS it is expedient to consolidate and amend the law regulating labour in factories; it is
hereby enacted as follows:__
CHAPTER I
PRELIMINARY
1. Short title, extent and commencement.__(1). This Act may be called the Factories Act, 1934.
2[(2) It extends to the whole of Pakistan].
(3) It shall come into force on the 1st day of January, 1935.
1For Statement of Objects and Reasons, see Gazette of India 1933, Pt. V, pp. 175 and 176; and for Report of Select Committee, see ibid., 1934, Pt. V, pp.44 and 45.
The Act has been applied to Phulera in the Excluded Area of Upper Tanawal to the extent the Act is applicable in N.W.F.P., subject to certain modifications, see N.W.F.P. (Upper
Tanawal) (Excluded Area) Laws Regulation, 1950, and extended to the Excluded Area of Upper Tanawal (N.W.F.P.) other than Phulera with effect from such date and subject to such
modifications as may be notified, see N.W.F.P. (Upper Tanawal) (Excluded Area) Laws Regulation, 1950.
The Act in its application in the Punjab has been amended by Factories (Punjab Amdt.) Act, 1940 (Punjab 7 of 1940), in N.W.F.P., by the Factories (NorthWest Frontier Province
Amdt.) Act, 1940 (N.W.F.P.7 of 1947),
This Act has been extended to the__
(i) Leased Areas of Baluchistan, see the Leased Areas (Laws) Order, 1950 (G.G.O.3 of 1950); and also applied in the Federated Areas of Baluchistan, see Gazette of India, 1937, Pt.I,
p.1499;
(ii) Baluchistan States Union, see the Baluchistan States Union (Federal Laws) (Extension) Order, 1953 (G.G.O.4 of 1953), as amended;
(iii) Khairpur State, see G.G.O.5 of 1953, as amended; and
(iv) State of Bahawalpur by the Bahawalpur (Extension of Federal Laws) Order, 1953 (G.G.O.11 of 1953), as amended.
The Act has been and shall be deemed to have been brought into force in Gwadur (with effect from the 18th September, 1958), by the Gwadur (Application of Central Laws)
Ordinance, 1960 (37 of 1960),s.2.
The Act has been applied to the Provincially Administered Tribal Areas or to the parts or those areas to which it does not already, apply see Regulation No. I of 1972, s.2 and Sch.
2Subs. by the Central Laws (Statute Reform) Ordinance, 1960 (21 of 1960) s.3 and 2nd Sch., (with effect from the 14th October, 1955), for the original subsection (2) as amended by
A.O., 1949, and the Federal Laws(Revision and Declaration) Act, 1951 (26 of 1951), s.8.
Page 5 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-265-320.jpg)
![2. Definitions.__ In this Act, unless there is anything repugnant in the subject or context,‑
(a) “adolescent” means a person who has completed his fifteenth but has not completed his
seventeenth year ;
(b) “adult” means a person who has completed his seventeenth year ;
(c) “child” means a person who has not completed his fifteenth year ;
(d) “day” means a period of twentyfour hours beginning at midnight;
(e) “week” means a period of seven days beginning at midnight on Saturday night;
(f) “power” means electrical energy, and any other form of energy which is mechanically
transmitted and is not generated by human or animal agency ;
(g) “manufacturing process” means any process‑
(i) for making, altering, repairing, ornamenting, finishing or packing, or otherwise
treating any article or substance with a view to its use, sale, transport, delivery or
disposal, or
(ii) for pumping oil, water or sewage, or
(iii) for generating, transforming or transmitting power;
(h) “worker” means a person employed, 1[directly or through an agency] whether for wages
or not, in any manufacturing process, or in cleaning any part of the machinery or premises
used for a manufacturing process, or in any other kind of work whatsoever incidental to
or connected with the manufacturing process or connected with the subject of the
manufacturing process, but does not include any person solely employed in a clerical
capacity in any room or place where no manufacturing process is being carried on;
(j) “factory” means any premises, including the precincts thereof whereon 2[ten] or more
workers are working, or were working on any day of the preceding twelve months, and in
any part of which a manufacturing process is being carried on 3[or is ordinarily carried on
whether with or without the aid of power];
but does not include a mine subject to the operation of the Mines Act, 1923 (IV of 1923).
(k) “machinery” includes all plant whereby power is generated, transformed, transmitted or
applied;
1Ins., by the Factories (Amdt) Act, 1973 (16 of 1973), s. 2 (w.e. f. 7th February, 1973).
2Subs., ibid for “twenty”,
3Subs., ibid., for certain words.
Page 6 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-266-320.jpg)
![(l) “occupier” of a factory means the person who has ultimate control over the affairs of the
factory:
Provided that where the affairs of a factory are entrusted to a managing agent, such
agent shall be deemed to be the occupier of the factory;
(m) where work of the same kind is carried out by two or more sets of workers working
during different periods of the day, each of such sets is called a “relay”, and the period or
periods for which it works is called a “shift”; and
(n) “prescribed” means prescribed by rules made by the 1[Provincial Government] under this
Act.
3. References to time of day. Reference to time of day in this Act are references__ 2* * * 3*
*, to 4* Standard Time, which is five 4* * * hours ahead of Greenwich Mean Time,5*
6* * * * * * *
Provided that for any area 7* * * in which 4* Standard Time is not ordinarily observed the
8[Provincial Government] may make rules__
(i) specifying the area,
(ii) defining the local mean time ordinarily observed therein, and
(iii) permitting such time to be observed in all or any of the factories situated in the area.
1Subs. by A. O., 1937, for “L. G.”.
2The brackets, letter and words “(a) in British India” omitted by A. O., 1949, (w. e. f. 28th March, 1949).
3The words “excluding Burma” omitted by A. O., 1937.
4The words “Indian” and “and a half” omitted by the Factories (Amdt.) Act, 1973 (16 of 1973) (w. e. f. 7th Feburary, 1973), s. 3.
5The word “and” omitted by A.O., 1949.
6Clause (b) omitted by A.O., 1937.
7The words “in British India” omitted by A. O. 1949.
8Subs. by A.O., 1937, for “L.G.”.
Page 7 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-267-320.jpg)
![4. Seasonal factories.___(1) For the purposes of this Act, a factory which is exclusively engaged
in one or more of the following manufacturing processes, namely, cotton ginning, cotton or jute
pressing, the decortication of groundnuts, the manufacture of coffee, indigo, lac, rubber, sugar
(including gur) or tea, or any manufacturing process which is incidental to or connected with any of
the aforesaid processes, is a seasonal factory:
(1) Provided that the 1[Provincial Government] may, by notification in the 2[official Gazette],
declare any such factory in which manufacturing processes are ordinarily carried on for more than
one hundred and eighty working days in the year, not to be a seasonal factory for the purposes of this
Act.
(2) The 1[Provincial Government] may, by notification in the 2[official Gazette], declare any
specified factory in which manufacturing processes are ordinarily carried on for not more than one
hundred and eighty working days in the year and cannot be carried on except during particular
seasons or at times dependent on the irregular action of natural forces, to be a seasonal factory for the
purposes of this Act.
3[5. Power to apply provisions applicable to factories to certain other places.__ (1) The
Provincial Government may, by notification in official Gazette, declare that all or any of the
provisions of this Act applicable to factories shall apply to any place wherein a manufacturing process
is being carried on or is ordinarily carried on whether with or without the use of power whenever
4[five] or more workers are working therein or have worked therein any one day of the twelve
months immediately preceding.
(2) A notification under sub‑section (1) may be made in respect of any one such place or in
respect of any class of such places or generally in respect of all such places.
(3) Notwithstanding anything contained in clause (j) of section 2, a place, to which all or any of
the provisions of this Act applicable to factories are for the time being applicable in pursuance of a
declaration under sub‑section (1), shall, to the extent to which such provisions are so made applicable
but not otherwise, be deemed to be a factory.]
6. Power to declare departments to be separate factories.__The 1[Provincial Government]
may, by order in writing, direct that the different departments or branches of a specified factory shall
be treated as separate factories for all or any of the purposes of this Act.
7. Power to exempt on a change in the factory.__ Where the 1[Provincial Government] is
satisfied that, following upon a change of occupier of a factory or in the manufacturing processes
carried on therein, the number of workers for the time being working in the factory is less than twenty
and is not likely to be twenty or more on any day during the ensuing twelve months, it may, by order
in writing, exempt such factory from operation of this Act:
Provided that any exemption so granted shall cease to have effect on and after any day on which
twenty or more workers work in the factory.
1Subs.by A. O., 1937, for “L.G.”.
2Subs. ibid. for “local official Gazette”.
3Subs. by the Factories (Amdt.) Act, 1941 (16 of 1941), s. 2, for the original section 5.
4Subs. by the Factories (Amdt.) Act 16 of 1973, s. 4, for “ten”.
Page 8 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-268-320.jpg)
![1[7A. Exemption from certain provisions of the Act. The provisions of section 14, clause (b) of
sub‑section (1) of section 15 sections 16, 17, 18, 21, 22, 23, 25 and subsection (3) of section 33Q
shall not apply in the first instance to any factory wherein not more than nineteen workers are
working or were working on any one of the twelve months immediately proceeding:
Provided that the Provincial Government may, by notification in the official Gazette, apply all or
any of the said provisions to any such factory or any class of such factories.]
8. Power to exempt during public emergency. In any case of public emergency the
2[Provincial Government] may, by notification in the 3[official Gazette], exempt any factory from
any or all of the provisions of this Act for such period as 4[it] may think fit.
9. Notice to Inspector before commencement of work.__ (1) Before work is begun in any
factory after the commencement of this Act, or before work is begun in any seasonal factory each
season, the occupier shall send to the Inspector a written notice containing‑
(a) the name of the factory and its situation,
(b) the address to which communications relating to the factory should be sent,
(c) the nature of the manufacturing processes to be carried on in the factory,
(d) the nature and amount of the power to be used, 5*
(e) the name of the person who shall be the manager of the factory for the purposes of this
Act, 6[and
(f) such other particulars as may be prescribed for the purposes of this Act].
7[(1‑A) In respect of all factories which come within the scope of this Act for the first time on the
commencement of the Factories (Amendment) Ordinance, 1972 (XLII of 1972) the occupier shall
send a written notice to the Inspector containing particulars specified in sub‑section (1) within thirty
days of such commencement.]
(2) Whenever another person is appointed as manager, the occupier shall send to the Inspector a
written notice of the change, within seven days from the date in which the new manager assumes
charge.
(3) During any period for which no person has been designated as manager of a factory under this
section, or during which the person designated does not manage the factory, any person found acting
as manager, or, if no such person is found, the occupier himself, shall be deemed to be the manager of
the factory for the purposes of this Act.
1Ins. by the Factories (Amdt.) Act, 1973 (16 of 1973), s. 5.
2Subs. by A. O. 1937, for “G.G. in C.”.
3Subs. ibid., for "Gazette of India".
4Subs. ibid., for "he".
5The word “and” at the end of clause (d) omitted by the Factories (Amdt.) Act, 1944 (14 of 1944), s. 2.
6The word “and” and cl. (f) added by the Factories (Amdt.) Act, 1944 (14 of 1944), s. 2.
7Ins. by the Factories (Amdt.) Act, 1973 (16 of 1973), s. 6.
Page 9 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-269-320.jpg)
![CHAPTER II
THE INSPECTING STAFF
10. Inspectors.__ (1) The 1[Provincial Government] may, by notification in the 2[official
Gazette], appoint such persons as it thinks fit to be Inspectors for the purposes of this Act within such
local limits as it may assign to them respectively.
(2) The 1[Provincial Government] may, by notification as aforesaid, appoint any person to be a
Chief Inspector, who shall, in addition to the powers conferred on a Chief Inspector under this Act,
exercise the powers of an Inspector throughout the Province.
(3) No person shall be appointed to be an Inspector under sub‑section (1) or a Chief Inspector
under sub‑section (2) or, having been so appointed, shall continue to hold office, who is or becomes
directly or indirectly interested in a factory or in any process or business carried on therein or in any
patent or machinery connected therewith.
(4) Every District Magistrate shall be an Inspector for his district.
(5) The 1[Provincial Government] may also, by notification as aforesaid, appoint such public
officers as it thinks fit to be additional Inspectors for all or any of the purposes of this Act, within
such local limits as it may assign to them respectively.
(6) In any area where there are more Inspectors than one, the 1[Provincial Government] may, by
notification as aforesaid, declare the powers which such Inspector shall respectively exercise, and the
Inspector to whom the prescribed notices are to be sent.
(7) Every Chief Inspector and Inspector shall be deemed to be a public servant within the
meaning of the Pakistan Penal Code (XLV of 1860) and shall be officially subordinate to such
authority as the 1[Provincial Government] may specify in this behalf.
11. Powers of Inspector. Subject to any rules made by the 1[Provincial Government] in this
behalf, an Inspector may, within the local limits for which he is appointed,‑
(a) enter, with such assistants (if any), being persons 3[in the service of the State] or of any
municipal or other public authority, as he thinks fit, any place which is, or which he has
reason to believe to be, used as a factory or capable of being declared to be a factory
under the provisions of section 5;
1Subs by A. O.,1937 for “L.G.”.
2Subs. ibid., for “local official Gazette”.
3The original words “in the employment of Government”, were first subs. by A. O., 1937 and then amended by A. O., 1961, Art. 2 and Sch. (with effect from the 23 March, 1956), to
read as above.
Page 10 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-270-320.jpg)
![(b) make such examination of the premises and plant and of any prescribed registers, and take
on the spot or otherwise such evidence of any persons as he may deem necessary for
carrying out the purposes of this Act; and
(c) exercise such other powers as may be necessary for carrying out the purposes of this
Act:
Provided that no one shall be required under this section to answer any question or give any
evidence tending to criminate himself.
12. Certifying surgeons.__(1) The 1[Provincial Government] may appoint such registered
medical practitioners as it thinks fit to be certifying surgeons for the purposes of this Act within such
local limits as it may assign to them respectively.
(2) A certifying surgeon may authorise any registered medical practitioner to exercise any of his
powers under this Act:
Provided that a certificate of fitness for employment granted by such authorised practitioner shall
be valid for a period of three months only, unless it is confirmed by the certifying surgeon himself
after examination of the person concerned.
Explanation.‑ In this section a “registered medical practitioner” means any person registered 2* *
* under any Act of 3[the 4[Federal] Legislature or any Provincial Legislature (21 & 22 Vict., c 90.)]
providing for the maintenance of a register of medical practitioners, and includes, in any area where
no such register is maintained, any person declared by the 5[Provincial Government], by notification
in the 6[official Gazette], to be a registered medical practitioner for the purposes of this section.
1 Subs. by A.O., 1937, for "L.G.".
2 Certain words omitted by the Federal Laws (Revision and Declaration) Ordinance, 1981 (27 of 1981), s. 3 and Sch., II.
3Subs. by A.O., 1949 (w.e.f. 28th March, 1949), for certain words.
4 Subs. by F.A.O., 1975, Art. 2 and Table (w.e.f. 28th July, 1975), for "Central".
5Subs. by A.O., 1937 (w.e.f. 1st April, 1937), for "L.G.".
6Subs. ibid., for "Local official Gazette".
Page 11 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-271-320.jpg)





![23. Precaution against contagious or infectious disease.__ (1) Each worker in a factory shall be
provided with a 'Hygiene Card' in which during 1[the months of January and July every year entries]
shall be recorded after examination by appointed factory doctor to the effect that the worker is not
suffering from any contagious or infectious disease, the fee of such an examination shall be fixed by
the Provincial Government and will be borne by the occupier or manager of the factory.
(2) If a worker is found to be suffering from any contagious or infectious disease on an
examination under sub‑section (1), he shall not be appointed on work till he is declared free of such a
disease.
2[23A. Compulsory vaccination and inoculation.__ Each worker in a factory shall be
vaccinated and inoculated against such diseases and at such intervals as may be prescribed. The
expenses, if any, of such vaccination and inoculation shall be borne by the occupier or manager of the
factory.]
24. Power to make rules for provision of canteens.__(1) The Provincial Government may make
rules requiring that in any specified factory wherein more than two hundred and fifty workers are
ordinarily employed, an adequate canteen shall be provided for the use of the workers.
(2) Without prejudice to the generality of the foregoing power such rules may provide for__
(a) the date by which such canteen shall be provided;
(b) the standards in respect of construction, accommodation, furniture and other equipment
of the canteen;
(c) the foodstuffs to be served therein and the charges which may be made, therefor ;
(d) representation of the workmen in the management of the canteens ;
(e) enabling, subject to such conditions, if any, as may be specified, the power to make rules
under clause (c) to be exercised also by the Chief Inspector.
3[24A. Welfare Officer.__ In every factory wherein not less than five hundred workers are
ordinarily employed, the occupier or manager shall employ such number of welfare Officers, having
such qualifications, to perform such duties and on such terms and conditions as may be prescribed.]
1Subs. by the Factories (Amdt.) Act 1973 (16 of 1973), s. 7, for “first fortnight of every calendar month and entry”.
2Ins. ibid., ss. 8 and 9.
3Ins. by the Factories (Amdt.) Act, 1973 (16 of 1973), ss. 8 and 9.
Page 17 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-277-320.jpg)


![(2) No woman or child shall be allowed in any factory to clean, lubricate or adjust any part of
machinery while that part is in motion, or to work between moving parts or between fixed and
moving parts of any machinery which is in motion.
(3) The Provincial Government may, by notification in the official Gazette, prohibit, in any
specified factory or class or description of factories, the cleaning, lubricating or adjusting by any
person, of specified parts of machinery when those parts are in motion.
28. Employment of young persons on dangerous machines.__ (1) No 1[child or adolescent]
shall work at any machine unless he has been fully instructed as to the dangers arising in connection
with the machine and the precautions to be observed and__
(a) has received sufficient training in work at the machine, or
(b) is under adequate supervision by a person who has thorough knowledge and experience of
the machine.
(2) This section shall apply to such machines as may be notified by the Provincial Government to
be of such a dangerous character that 2[children or adolescents] ought not to work at them unless the
foregoing requirements are complied with.
29. Striking gear and devices for cutting off power.___(1) In every factory‑
(a) suitable striking gear or other efficient mechanical appliances shall be provided and
maintained and used to move driving belts to and from fast and loose pulleys which form
part of the transmission machinery, and such gear or appliances shall be so constructed,
placed and maintained as to prevent the belt from creeping back on the fast pulleys ;
(b) driving belts when not in use shall not be allowed to rest or ride upon shafting in motion.
(2) In every factory suitable devices for cutting off power in emergencies from running machinery
shall be provided and maintained in every workroom.
(3) In respect of factories in operation before the commencement of this Ordinance3 the
provisions of sub‑section (2) shall apply only to workrooms in which electricity is used for power.
30. Self‑acting machines. No traversing part of a self_acting machine in any factory and no
material carried thereon shall, if the space over which it runs is a space over which any person is
liable to pass whether in the course of his employment or otherwise be allowed to run on its outward
or inward traverse within a distance of eighteen inches from any fixed structure which is not part of
the machine:
1Subs. by the Factories (Amdt.) Act, 1973 (16 of 1973), s. 10, for “young person”.
2Subs. ibid., for “young persons”.
3I.e., the Labour Laws (Amendment) Ordinance, 1972 (9 of 1972).
Page 20 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-280-320.jpg)








![33‑Q. Additional power to make health and safety rules relating to shelters during rest.__
(1) The Provincial Government may make rules requiring that in any specified factory wherein more
than one hundred and fifty workers are ordinarily employed, an adequate shelter shall be provided for
the use of workers during periods of rest, and such rules may prescribe the standards of such shelters.
(2) rooms for children.__ The Provincial Government may also make rules__
(a) requiring that in any specified factory, wherein more than fifty women workers are
ordinarily employed, a suitable room shall be reserved for the use of children under the
age of six years belonging to such women, and
(b) prescribing the standards for such rooms and the nature of the supervision to be exercised
over the children therein.
(3) certificates of stability.__ The Provincial Government may also make rules, for any class of
factories and for the whole or any part of the Province, requiring that work on a manufacturing
process carried on with the aid of power shall not be begun in any building or part of a building
erected or taken into use as a factory after the commencement of this Act, until a certificate of
stability in the prescribed form signed by a person possessing the prescribed qualifications, has been
sent to the Inspector.
(4) hazardous operations.__ Where the Provincial Government is satisfied that any operation in
a factory exposes any persons employed upon it to a serious risk of bodily injury, poisoning or
disease, it may make rules applicable to any factory or class of factories in which the operation is
carried on‑
(a) specifying the operation and declaring it to be hazardous,
(b) prohibiting or restricting the employment of women, adolescents or children upon the
operation.
(c) providing for the medical examination of persons employed or seeking to be employed
upon the operation and prohibiting the employment of persons not certified as fit for such
employment, and
(d) providing for the protection of all persons employed upon the operation or in the vicinity
of the places where it is carried on.”.
1[(5) The Provincial Government may also make rules requiring the occupiers or managers of
factories to maintain stores of firstaid appliances and provide for their proper custody and use.]
1 Added by the Factories (Amdt.) Act, 1973 (16 of 1973), s. 11.
Page 29 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-289-320.jpg)
![CHAPTER IV
RESTRICTIONS ON WORKING HOURS OF ADULTS
134. Weekly hours. No adult worker shall be allowed to work in a factory for more than 2[forty
eight] hours in any week, or, where the factory is a seasonal one, for more than 3[fifty] hours in any
week:
Provided that an adult worker in a 4*factory engaged in work which for technical reasons must be
continuous throughout the day may work for fiftysix hours in any week.
35. Weekly holiday.__(1) No adult worker shall be allowed to work in a factory on a Sunday
unless__
(a) he has had or will have a holiday for a whole day on one of the three days immediately
before or after that Sunday, and
(b) the manager of the factory has, before that Sunday or the substituted day, whichever is
earlier,__
(i) delivered a notice to the office of the Inspector of his intention to require the worker
to work on the Sunday and of the day which is to be substituted, and
(ii) displayed a notice to that effect in the factory :
Provided that no substitution shall be made which will result in any worker working for more than
ten days consecutively without a holiday for a whole day.
(2) Notices given under subsection (1) may be cancelled by a notice delivered to the office of the
Inspector and a notice displayed in the factory not later than the day before the Sunday or the holiday
to be cancelled, whichever is earlier.
(3) Where, in accordance with the provisions of sub‑section (1), any worker works on a Sunday
and has had a holiday on one of the three days immediately before it, that Sunday shall, for the
purpose of calculating his weekly hours of work, be included in the preceding week.
1For Notifin. under this section, see Gazette of Karachi, 1958, Pt. II, p. 52.
2Subs. by the Factories (Amdt.) Act 1946 (10 of 1946), s. 2 (with effect from the Ist August, 1946), for “fiftyfour”
3Subs.ibid. for “sixty”.
4 The word “nonseasonal” omitted by the Factories (Amdt.) Act, 1947 (5 of 1947), s. 3.
Page 30 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-290-320.jpg)
![1[35‑A. Compensatory holidays.__(1) Where, as a result of the passing of an order or the
making of a rule under the provisions of this Act exempting a factory or the workers therein from the
provisions of section 35, a worker is deprived of any of the weekly holidays for which provision is
made by sub‑section (1) of that section, he shall be allowed, as soon as circumstances permit,
compensatory holidays of equal number to the holidays so lost.
(2) The Provincial Government may make rules prescribing the manner in which the holidays, for
which provision is made in sub‑section (1), shall be allowed.]
236. Daily hours. No adult worker shall be allowed to work in a factory for more than 3[nine]
hours in any day:
Provided that a male adult worker in a seasonal factory may work for 4[ten] hours in any day.
37. Intervals for rest. The periods of work of adult workers in a factory during each day shall be
fixed either‑
(a) so that no period shall exceed six hours, and so that no worker shall work for more than
six hours before he has had an interval for rest of at least one hour; or
(b) so that no period shall exceed five hours and so that no worker shall work for more than
five hours before he has had an interval for rest of at least half an hours, or for more than
eight and a half hours before he has had at least two such intervals.
1S.35A ins. by the Factories (Amdt.) Act, 1945 (3 of 1945), s. 2. (with effect from 1st January 1946).
2For Notifn., under this section, see Gazette of Karachi, 1958, Pt. II, p. 52.
3Subs. by the Factories (Amdt.) Act 1946 (10 of 1946), s. 3 (with effect from the Ist August, 1946), for “ten”
4Subs. Ibid., for “eleven”.
Page 31 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-291-320.jpg)
![138. Spread over. The periods of work of an adult worker in a factory shall be so arranged that
alongwith his intervals for rest under section 37, they shall not spread over more than 2[twelve] hours,
or where the factory is a seasonal one, eleven and a half] hours in any day, save with the permission
of the 3[Provincial Government] and subject to such conditions as it may impose, either generally or
in the case of any particular factory.
39. Notice of Periods for Work for Adults and preparation thereof.__ (1) There shall be
displayed and correctly maintained in every factory in accordance with the provisions of sub‑section
(2) of section 76 a Notice of Periods for Work for Adults showing clearly the periods within which
adult workers may be required to work.
(2) The periods shown in the notice required by sub‑section (1) shall be fixed before hand in
accordance with the following provisions of this section and shall be such that workers working for
those periods would not be working in contravention of any of the provisions of sections 34, 35, 36,
37 and 38.
(3) Where all the adult workers in a factory are required to work within the same periods, the
manager of the factory shall fix those periods for such workers generally.
(4) Where all the adult workers in a factory are not required to work within the same periods, the
manager of the factory shall classify them into groups according to the nature of their work.
(5) For each group which is not required to work on a system of shifts, the manager of the factory
shall fix the periods within which the group may be required to work.
(6) Where any group is required to work on a system of shifts and the relays are not to be subject
to predetermined periodical changes of shift, the manager of the factory shall fix the periods within
which each relay of the group may be required to work.
(7) Where any group is to work on a system of shifts and the relays are to be subject to
predetermined periodical changes of shifts, the manager of the factory shall draw up a scheme of
shifts whereunder the periods within which any relay of the group may be required to work and the
relay which will be working at any time of the day shall be known for any day.
(8) The 4[Provincial Government] may make rules prescribing forms for the Notice of Periods for
Work for Adults and the manner in which it shall be maintained.
40. Copy of Notice of Periods for Work to be sent to Inspector._(1) A copy of the Notice
referred to in sub‑section (1) of section 39 shall be sent in duplicate to the Inspector within fourteen
days after the commencement of this Act, or, if the factory begins work after the commencement of
this Act, before the day on which it begins work.
(2) Any proposed change in the system of work in a factory which will necessitate a change in the
Notice shall be notified to the Inspector in duplicate before the change is made, and, except with the
previous sanction of the Inspector, no such change shall be made until one week has elapsed since the
last change.
1For Notifn., under this section, see Gazette of Karachi, 1958, Pt. II, p. 52.
2Subs. by Act III of 06, s. 3.
3Subs. by A. O., 1937, for “L. G.”.
4Subs. by A. O., 1937, for “L.G.”.
Page 32 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-292-320.jpg)
![41. Register of Adult Workers.__ (1) The manager of every factory shall maintain a Register of
Adult Workers showing__
(a) the name 1[and age] of each adult worker in the factory,
(b) the nature of his work,
(c) the group, if any, in which he is included,
(d) where his group works on shifts, the relay to which he is allotted, and
(e) such other particulars as may be prescribed:
Provided that, if the Inspector is of opinion that any muster roll or register maintained as part of
the routine of a factory gives in respect of any or all of the workers in the factory the particulars
required under this section, he may, by order in writing, direct that such muster roll or register shall to
the corresponding extent be maintained in place of and be treated as the Register of Adult Workers in
that factory:
Provided further that, where the 2[Provincial Government] is satisfied that the conditions of work
in any factory or class of factories are such that there is no appreciable risk of contravention of the
provisions of this Chapter in the case of that factory or factories of that class, as the case may be, the
2[Provincial Government] may, by written order, exempt, on such conditions as it may impose, that
factory or all factories of that class, as the case may be, from the provisions of this section.
(2) The 2[Provincial Government] may make rules prescribing the form of the Register of Adult
Workers, the manner in which it shall be maintained and the period for which it shall be preserved.
42. Hours of work to correspond with Notice under section 39 and Register under section
41. No adult worker shall be allowed to work otherwise than in accordance with the Notice of Periods
for Work for Adults displayed under subsection (1) of section 39 and the entries made beforehand
against his name in the Register of Adult Workers maintained under section 41.
1Ins. by the Factories (Amdt.) Act, 1973(16 of 1973), s.12.
2Subs. by A. O., 1937, for “L.G.”.
Page 33 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-293-320.jpg)
![43. Powers to make rules exempting from restrictions.__(1) The 1[Provincial Government]
may make rules defining the persons who hold positions of supervision or management or are
employed in a confidential position in a factory, and the provisions of this Chapter, 2[other than the
provisions of clause (b) of sub‑section (1) of section 45 and of the provisos to that subsection], shall
not apply to any person so defined.
(2) The 1[Provincial Government] may make rules for adult workers providing for the
exemption3, to such extent and subject to such conditions as may be prescribed in such rules,__
(a) of workers engaged on urgent repairs__ from the provisions of sections 34, 35, 36, 37 and
38 ;
(b) of workers engaged in work in the nature of preparatory or complementary work which
must necessarily be carried on outside the limits laid down for the general working of the
factory__from the provisions of sections 34, 36, 37 and 38 ;
(c) of workers engaged in work which is necessarily so intermittent that the intervals during
which they do not work while on duty ordinarily amount to more than intervals for rest
required under section 37 from the provisions of sections 34, 36, 37 and 38;
(d) of workers engaged in any work which for technical reasons must be carried on
continuously throughout the day__from the provisions of sections 34, 35, 36, 37 and 38;
(e) of workers engaged in making or supplying articles of prime necessity which must be
made or supplied every day__from the provisions of section 35;
(f) of workers engaged in a manufacturing process which cannot be carried on except during
fixed seasons__from the provisions of section 35 ;
(g) of workers engaged in a manufacturing process which cannot be carried on except at
times dependent on the irregular action of natural forces__from the provisions of section
35 and section 37; and
(h) of workers engaged in enginerooms or boiler‑houses from the provisions of section 35.
(3) Rules made under sub‑section (2) providing for any exemption may also provide for any
consequential exemption from the provisions of sections 39 and 40 which the 1[Provincial
Government] may deem to be expedient, subject to such conditions as it may impose.
(4) In making rules under this section the 1[Provincial Government] shall prescribe the maximum
limits for the weekly hours of work for all classes of workers, and any exemption given, other than an
exemption under clause (a) of sub‑section (2), shall be subject to such limits.
(5) Rules made under this section shall remain in force for not more than three years.
1Subs. by A. O., 1937, for “L.G.”.
2Ins. by the Factories (Amdt.) Act, 1935, (11 of 1935), s. 2.
3For the Karachi Factories (Adult Exemption) Rules, 1958, see Gazette of Karachi, 1959, Pt. II, pp. 8390.
Page 34 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-294-320.jpg)
![44. Power to make orders exempting from restrictions.__ (1) Where the 1[Provincial
Government] is satisfied that, owing to the nature of the work carried on or to other circumstances, it
is unreasonable to require that the periods of work of any adult workers in any factory or class of
factories should be fixed beforehand, it may, by written order, relax or modify the provisions of
sections 39 and 40 in respect of such workers to such extent and in such manner as it may think fit,
and subject to such conditions as it may deem expedient to ensure control over periods of work.
(2). The 1[Provincial Government], or subject to the control of the 1[Provincial Government] the
Chief Inspector, may, by written order2, exempt, on such conditions as it or he may deem expedient,
any or all of the adult workers in any factory, or group or class of factories, from any or all of the
provisions of sections 34, 35, 36, 37, 38, 39 and 40, on the ground that the exemption is required to
enable the factory or factories to deal with an exceptional press of work.
(3) Any exemption given under sub‑section (2) in respect of weekly hours of work shall be
subject to the maximum limits prescribed under sub‑section (4) of section 43.
3[(4) An order under sub‑section (2) shall remain in force for such period, not exceeding two
months from the date on which notice thereof is given to the manager of the factory, as may be
specified in the order:
Provided that if in the opinion of the Provincial Government the public interest so requires, the
Provincial Government may from time to time, by notification in the official Gazette, extend the
operation of any such order for a period, not exceeding six months at any one time, as may be
specified in the notification.]
45. Further restrictions on the employment of women.__(1) The provisions of this Chapter
shall, in their application to women workers in factories, be supplemented by the following further
restrictions, namely__
(a) no exemption from the provisions of section 36 may be granted in respect of any woman;
and
(b) no woman shall be allowed to work in a factory except between 6 A. M. and 7 P.M. :
1Subs. by A. O. 1937, for “L.G.”.
2For an instance of such Order, see the Punjab Gazette, 1951, Pt.I,p. 87
3Subs. by the Factories (Amdt.) Act, 1946 (10 of 1946), s. 5 (with effect from 1st August, 1946), for original subsection (4).
Page 35 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-295-320.jpg)
![1[Provided that if the employer arranges for the transport facilities, a woman with her consent
may work up to 10.00 p.m. in two shifts.]
(2) The 2[Provincial Government] may make rules providing for the exemption from the above
restrictions, to such extent and subject to such conditions as it may prescribe, of women working in
fishcuring or fishcanning factories where the employment of women beyond the said hours is
necessary to prevent damage to or deterioration in any raw material.
(3) Rules made under sub‑section (2) shall remain in force for not more than three year.
46. Special provision for nightshifts. Where a worker works on a shift which extends over
midnight, the ensuing day for him shall be deemed to be the period of twentyfour hours beginning
when such shift ends, and the hours he has worked after midnight shall be counted towards the
previous day:
Provided that the 2[Provincial Government] may, by order in writing, direct that in the case of
any specified factory or any specified class of workers therein the ensuing day shall be deemed to be
the period of twentyfour hours beginning when such shift begins and that the hours worked before
midnight shall be counted towards the ensuing day.
47. Extra pay for overtime.__ 3[(1) Where a worker__
(a) in a non‑seasonal factory works for more than nine hours in any day or for more than
fortyeight hours in any week, or
(b) in a seasonal factory works for more than nine hours in any day or for more than fifty
hours in any week,‑
he shall be entitled in respect of the overtime worked to pay at the rate of twice his ordinary rate
of a pay.]
4[Explanation. In this sub‑section, ‘ordinary rate of pay’ means all remuneration capable of
being expressed in terms of money which would, if the terms of the contract of employment, express
or implied, were fulfilled, be payable to a worker in respect of his employment or of work done in
such employment, but does not include__
(i) the value of any houseaccommodation, supply of light, water, medical attendance or other
amenity ;
(ii) any contribution paid by the employer to any pension fund or provident fund ;
(iii) any travelling allowance or the value of travelling concession ; or
(iv) any gratuity, bonus or share in the profits of the factory.]
1Subs. & omitted by Act III of 06, s. 3.
2Subs. by A. O., 1937, for “L.G.”.
3Subs. by the Factories (Amdt.) Act, 1946 (10 of 1946). s. 7, for the original sub‑sections (1) and (2) (with effect from 1st August, 1946).
4Explanation added the Factories (Amdt.) Act, 1973 (16 of 1973), s. 13.
Page 36 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-296-320.jpg)
![(3) Where any workers are paid on a piece rate basis, the 1[Provincial Government] in
consultation with the industry concerned may for the purposes of this section fix time rates as nearly
as possible equivalent to the average rate of earnings of those workers, and the rates so fixed shall be
deemed to be the ordinary rates of pay of those workers for the purposes of this section.
(4) The 1[Provincial Government] may prescribe the registers that shall be maintained in a factory
for the purpose of securing compliance with the provisions of this section.
48. Restriction on double employment. No adult worker shall be allowed to work in any factory
on any day on which he has already been working in any other factory, save in such circumstances as
may be prescribed.
49. Control of overlapping shifts. The 1[Provincial Government] may make rules providing that
in any specified class or classes of factories work shall not be carried on by a system of shifts so
arranged that more than one relay of workers is engaged in work for the same kind at the same time
save with the permission of the 1[Provincial Government] and subject to such conditions as it may
impose, either generally or in the case of any particular factory.
_____________
2[CHAPTER IV A
HOLIDAYS WITH PAY
49‑A. Application of Chapter.__(1) The provisions of this Chapter shall not apply to a seasonal
factory.
(2) The provisions of this Chapter shall not operate to the prejudice of any rights to which a
worker may be entitled under any other enactment, or under the terms of any award, agreement or
contract of service.
1Subs. by A.O., 1937, for "L.G.".
2Chapter IV A ins. by the Factories (Amdt.) Act, 1945 (3 of 1945), s. 3 (with effect from the 1st January, 1946).
Page 37 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-297-320.jpg)

![49G. Exemption of factories from provisions of this Chapter. Where the Provincial
Government is satisfied that the leave rules applicable to workers in a factory provide benefits
substantially similar to those for which this Chapter makes provision, it may, by written order, exempt
the factory from the provisions of this Chapter.]
1[49H. Casual leave and sicks leave.__ (1) Every worker shall entitled to casual leave with full
pay for ten days in a year.
(2) Every worker shall be entitled to sixteen days, sick leave on half average pay in a year.
49I. Festival holidays.__(1) Every worker shall be allowed holidays with pay on all days declared
by the Provincial Government to be festival holidays.
(2) A worker may be required to work on any festival holiday but one day additional
compensatory holiday with full pay and a substitute holiday shall be allowed to him in accordance
with the provisions of section 35.]
___________
CHAPTER V
SPECIAL PROVISIONS FOR ADOLESCENTS AND CHILDREN
50. Prohibition of employment of young children. No child who has not completed his
2[fourteenth] year shall be allowed to work in any factory.
51. Nonadult workers to carry tokens giving reference to certificates of fitness. No child
who has completed his 2[fourteenth] year and no adolescent shall be allowed to work in any factory
unless__
(a) a certificate of fitness granted to him under section 52 is in the custody of the manager of
the factory, and
(b) he carries while he is at work a token giving a reference to such certificate.
1Added by the Factories (Amdt.) Act, 1973 (16 of 1973), s. 15.
2Subs. by the Labour Laws (Amdt.), Act, 1977 (17 of 1977), s. 2 and First Schedule for “twelfth”.
Page 39 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-299-320.jpg)
![52. Certificates of fitness.__(1) A certifying surgeon shall, on the application of any 1[child or
adolescent] who wishes to work in a factory, or of the parent or guardian of such person, or of the
manager of the factory in which such person wishes to work, examine such person and ascertain his
fitness for such work.
(2) The certifying surgeon, after examination, may grant to such person, in the prescribed form,__
(a) a certificate of fitness to work in a factory as a child, if he is satisfied that such person has
completed his 2[fourteenth] year, that he has attained the prescribed physical standards (if
any), and that he is fit for such work; or
(b) a certificate of fitness to work in a factory as an adult, if he is satisfied that such person
has completed his fifteenth year and is fit for full day’s work in a factory.
(3) A certifying surgeon may revoke any certificate granted under sub‑section (2) if, in his
opinion, the holder of it is no longer fit to work in the capacity stated therein in a factory.
(4) Where a certifying surgeon or a practitioner authorised under sub‑section (2) of section 12
refuses to grant a certificate or a certificate of the kind requested, or revokes a certificate, he shall, if
so requested by any person who could have applied for the certificate, state his reasons in writing for
so doing.
53. Effect of certificate granted to adolescent.__ (1) An adolescent who has been granted a
certificate of fitness to work in a factory as an adult, under clause (b) of sub‑section (2) of section 52,
and who, while at work in a factory, carries a token giving reference to the certificate, shall be
deemed to be an adult for all the purposes of Chapter IV.
(2) An adolescent who has not been granted a certificate of fitness to work in a factory as an
adult under sub‑section (2) of section 52 shall, notwithstanding his age, be deemed to be a child for
the purposes of this Act.
54. Restrictions of the working hours of a child.__(1) No child shall be allowed to work in a
factory for more than five hours in any day.
(2) The hours of work of a child shall be so arranged that they shall not spread over more than
seven‑and‑a‑half hours in any day.
1Subs. by Act, 16 of 1973, s. 16. for “young person”.
2Subs. by the Labour Laws (Amdt.), Act, 1977 (17 of 1977), s. 2 and First Schedule for “twelfth”.
Page 40 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-300-320.jpg)
![(3) No child 1[or adolescent] shall be allowed to work in a factory except between 6 A. M. and 7
P.M. :
Provided that the 2[Provincial Government] may, by notification in the 3[official Gazette], in
respect of any class or classes of factories and for the whole year or any part of it, vary these limits to
any span of thirteen hours between 5 A. M. and 4[7‑30 P.M.].
(4) The provisions of section 35 shall apply also to child workers, but no exemption from the
provisions of that section may be granted in respect of any child.
(5) No child shall be allowed to work in any factory on any day on which he has already been
working in another factory.
55. Notice of Periods for Work for Children.__(1) There shall be displayed and correctly
maintained in every factory, in accordance with the provisions of sub‑section (2) of section 76, a
Notice of Periods for Work for Children, showing clearly the periods within which children may be
required to work.
(2) The periods shown in the Notice required by sub‑section (1) shall be fixed beforehand in
accordance with the method laid down for adults in section 39 and shall be such that children working
for those periods would not be working in contravention of section 54.
(3) The provisions of section 40 shall apply also to the Notice of Periods for Work for Children.
(4) The 2[Provincial Government] may make rules prescribing forms for the Notice of Periods for
Work for Children and the manner in which it shall be maintained.
56. Register of Child Workers.__ (1) The manager of every factory in which children are
employed shall maintain a Register of Child Workers showing__
(a) the name 5[and age] of each child worker in the factory
(b) the nature of his work,
(c) the group, if any, in which he is included,
1Ins. by the Factories (Amdt.) Act. 1973 (16 of 1973), s. 17.
2Subs. by A. O. 1937, for “L. G.”.
3Subs. ibid., for “local official Gazette”.
4For these figures and letters the figures and letters “830 P.M” were temporarily subs, for the duration of the war by the Factories (Amdt.), Act, 1944 (14 of 1944), s. 5.
5Ins. by the Labour Laws (Amdt), Act, 1973 (16 of 1973), s. 18.
Page 41 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-301-320.jpg)
![(d) where his group works on shifts, the relay to which he is allotted,
(e) the number of his certificate of fitness granted under section 52, and
(f) such other particulars as may be prescribed.
(2) The 1[Provincial Government] may make rules prescribing the form of the Register of Child
Workers, the manner in which it shall be maintained and the period for which it shall be preserved.
57. Hours of work to correspond with Notice and Register. No child shall be allowed to work
otherwise than in accordance with the Notice of Periods for Work for Children displayed under
sub‑section (1) of section 55 and the entries made before hand against his name in the Register of
Child Workers maintained under sub‑section (1) of section 56.
58. Power to require medical examination. Where an Inspector is of opinion—
(a) that any person working in a factory without a certificate of fitness is a child or an
adolescent, or
(b) that a child or adolescent working in a factory with a certificate is no longer fit to work in
the capacity stated therein,
he may serve on the manager of the factory a notice requiring that such person, or that such child
or adolescent, as the case may be, shall be examined by a certifying surgeon or by a practitioner
authorised under sub‑section (2) of section 12, and such person, child or adolescent shall not, if the
Inspector so directs, be allowed to work in any factory until he has been so examined and has
been granted a certificate of fitness or a fresh certificate of fitness, as the case may be.
59. Power to make rules. The 1[Provincial Government] may make rules__
(a) prescribing the forms of certificates of fitness to be granted under section 52, providing
for the grant of duplicates in the event of loss of the original certificates, and fixing the
fees which may be charged for such certificates and such duplicates;
(b) prescribing the physical standards to be attained by children and adolescents ;
(c) regulating the procedure of certifying surgeons under this Chapter, and specifying other
duties which they may be required to perform in connection with the employment of
children and adolescents in factories; and
(d) providing for any other matter which may be expedient in order to give effect to the
provisions of this Chapter.
1Subs, by A.O. ,1937, for “L. G.”.
Page 42 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-302-320.jpg)
![1[59A. Provisions to be in addition to Act XXVI of 1938.__The provisions of this Chapter shall
be in addition to, and not in derogation of, the provisions of the Employment of Children Act, 1938
(XXVI of 1938)] .
_________
CHAPTER VI
PENALTIES AND PROCEDURE
60. Penalty for contraventions of Act and rules. If in any factory—
(a) there is any contravention__
(i) of any of the provisions of sections 13 to 2[32] inclusive, or
(ii) of any order made under any of the said sections, or
(iii) of any of the said sections read with rules made in pursuance thereof under
3[section 33J] or
(iv) of any rules made under any of the said sections or under 4[sections 33J and 33Q],
or
(v) of any condition imposed under sub‑section (3) of section 5[33P], or
(b) any person is allowed to work in contravention__
(i) of any of the provisions of sections 34 to 38 inclusive, 42, 45, 6[48, 49H and 49I], or
(ii) of any rule made under any of the said sections, or under section 49, or
(iii) of any condition attached or any exemption granted under section 43 or section 44
or section 45 or to any permission granted under section 38 or section 49, or
(c) there is any contravention of any of the provisions of sections 39 to 41 inclusive or of any
rule made under section 39, section 41 or section 47, or of any condition attached to any
exemption granted under section 41 or to any modification or relaxation made under
section 44, or
1Subs. by the Factories (Amdt.), Act, 1973 (16 of 1973),s.19, for Chapter VA, which had been ins. by the Factories (Amdt.) Act, 1940 (17 of 1940) s.2 and amended by the Labour
Laws (Amdt.) Ordinance, 1972 (9 of 1972),s.2 and Ist. Sch. (w.e.f. 13th April, 1972).
2Subs. by the Labour Laws (Amdt.) Ordinance, 1972 (9 of 1972), s. 2 and First Schedule for “29”.
3Subs. ibid., for “clause (a) of section 32”.
4Subs. ibid., for "clause (b), clause (c) or clause (g) of section 32 or section “33”.
5Subs. ibid., for “31”.
6Subs. by the Laboure Laws (Amdt.) Act. 1975 (11 of 1975) s.2 and Sch. (w. e. f 25th January, 1975), for the word and figures “and 48”.
Page 43 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-303-320.jpg)
![(d) any person is not paid any extra pay to which he is entitled under the provisions of section
47, or
(e) any adolescent or child is allowed to work in contravention of any of the provisions of
sections 50, 51, 54, 55, 57 and 58, or
(f) there is any contravention of section 55 or section 56 or of any rules made under either of
these sections, or under clause (d) of section 59, 1[or]
2[(g) there is any contravention of section 49B, 49C, or 49D, or of any rule made under
section 49F,]
the manager and occupier of the factory shall each be punishable with fine which may extend to
five hundred rupees :
Provided that if both the manager and the occupier are convicted, the aggregate of the fines
inflicted in respect of the same contravention shall not exceed this amount.
61. Enhanced penalty in certain cases after previous conviction. If any person who has been
convicted of any offence punishable under clauses (b) to 3[(g)] inclusive of section 60 is again guilty
of an offence involving a contravention of the same provision, he shall be punishable on the second
conviction with fine which may extend to seven hundred and fifty rupees and shall not be less than
one hundred rupees, and if he is again so guilty, shall be punishable on the third or any subsequent
conviction with fine which may extend to one thousand rupees and shall not be less than two hundred
and fifty rupees:
Provided that for the purposes of this section no cognizance shall be taken of any conviction
made more than two years before the commission of the offence which is being punished :
Provided further that the Court, if it is satisfied that there are exceptional circumstances
warranting such a course, may, after recording its reasons in writing, impose a smaller fine than is
required by this section.
62. Penalty for failure to give notice of commencement of work or of change of manager. An
occupier of a factory who fails to give any notice required by sub‑section (1) 4[subsection (1A)] or
sub‑section 2 of section 9 shall be punishable with fine which may extend to five hundred rupees.
1The word “or” added by the Factories (Amdt.) Act, 1945 (3 of 1945), s. 4 (with effect from the 1st January, 1946).
2Cl. (g) ins ibid.
3Subs. by the the Factories (Amdt.) Act, 1945 (3 of 1945), s.5, for the brackets and the letter “(f)”.
4Ins. by the Factories (Amdt.) Act, 1973 (16 of 1973), s.20.
Page 44 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-304-320.jpg)
![63. Penalty for obstructing Inspector. Whoever willfully obstructs an Inspector in the exercise
of any power under section 11, or fails to produce on demand by an Inspector any registers or other
documents in his custody kept in pursuance of this Act or of any of the rules made thereunder, or
conceals or prevents any worker in a factory from appearing before or being examined by an
Inspector, shall be punishable with fine which may extend to five hundred rupees.
64. Penalty for failure to give notice of accidents. A manager of a factory who fails to give
notice of an accident as required under section 1[33‑N] shall be punishable with fine which may
extend to five hundred rupees.
65. Penalty for failure to make returns. If in respect of any factory any return is not furnished
as required under section 77, the manager and the occupier of the factory shall each be liable to fine
which may extend to five hundred rupees :
Provided that if both the manager and the occupier are convicted, the aggregate of the fines
inflicted shall not exceed this amount.
66. Penalty for smoking or using naked light in vicinity of inflammable material. Whoever
smokes, or uses a naked light or causes or permits any such light to be used in the vicinity of any
inflammable material in a factory shall be punishable with fine which may extend to five hundred
rupees.
Exception.__This provision does not extend to the use, in accordance with such precautions as
may be prescribed, of a naked light in the course of a manufacturing process.
67. Penalty for using false certificate. Whoever knowingly uses or attempts to use, as a
certificate granted to himself under section 52, a certificate granted to another person under that
section, or who, having procured such a certificate, knowingly allows it to be used, or an attempt to
use it to be made, by another person, shall be punishable with fine which may extend to twenty
rupees.
68. Penalty on guardian for permitting double employment of a child. lf a child works in a
factory on any day on which he has already been working in another factory, the parent or guardian
of the child or the person having custody of or control over him, or obtaining any direct benefit from
his wages, shall be punishable with fine which may extend to twenty rupees, unless it appears to the
Court that the child so worked without the consent, connivance or wilful default of such parent,
guardian or person.
69. Penalty for failure to display certain notices. A manager of a factory who fails to display
the notice required under sub‑section (1) of section 76 or by any rule made under this Act, or to
display or maintain any such notice as required by sub‑section (2) of that section, shall be punishable
with fine which may extend to five hundred rupees.
1Subs. by the Labour Laws (Amdt.), Ordinance, 1972 (9 of 1972), s. 2 and First Schedule for “30”.
Page 45 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-305-320.jpg)
![70. Determination of “occupier” for purposes of this Chapter.__ (1) Where the occupier of a
factory is a firm or other association of individuals, any one of the individual partners or members
thereof may be prosecuted and punished under this Chapter for any offence for which the occupier of
the factory is punishable:
Provided that the firm or association may give notice to the Inspector that it has nominated one of
its member who is resident in 1[Pakistan] to be the occupier of the factory for the purposes of this
Chapter, and such individual shall so long as he is so resident be deemed to be the occupier for the
purposes of this Chapter until further notice cancelling his nomination is received by the Inspector or
until he ceases to be a partner or member of the firm or association.
(2) Where the occupier of a factory is a company, any one of the directors thereof, or, in the case
of a private company, any one of the shareholders thereof, may be prosecuted and punished under
this Chapter for any offence for which the occupier of the factory is punishable:
Provided that the company may give notice to the Inspector that it has nominated a director, or, in
the case of a private company, a shareholder, who is resident in either case in1[Pakistan] to be the
occupier of the factory for the purposes of this Chapter, and such director or shareholder shall so long
as he is so resident be deemed to be the occupier of the factory for the purposes of this Chapter until
further notice cancelling his nomination is received by the Inspector or until he ceases to be director
or shareholder.
71. Exemption of occupier or manager from liability in certain cases.__ (1) Where the
occupier or manager of a factory is charged with an offence against this Act, he shall be entitled upon
complaint duly made by him to have any other person whom he charges as the actual offender
brought before the Court at the time appointed for hearing the charge; and if, after the commission of
the offence has been proved, the occupier or manager of the factory proves to the satisfaction of the
Court__
(a) that he has used due diligence to enforce the execution of this Act, and
(b) that the said other person committed the offence in question without his knowledge,
consent or connivance,
that other person shall be convicted of the offence and shall be liable to the like fine as if he were
the occupier or manager and the occupier or manager, shall be discharged from any liability under this
Act.
(2) When it is made to appear to the satisfaction of the Inspector at any time prior to the
institution of the proceedings__
(a) that the occupier of manager of the factory has used all due diligence to enforce the
execution of this Act, and
(b) by what person the offence has been committed, and
1Subs. by the Central Laws (Statue Reform) Ordinance, 1960 (21 of 1960), s. 3 and 2nd Sch. (with effect from 14th October, 1955), for “the Provinces and the Capital of the
Federation” which had been subs. by A. O., 1949, for “British India”.
Page 46 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-306-320.jpg)

![CHAPTER VII
SUPPLEMENTAL
76 Display of factory notices.___(1) In addition to the notices required to be displayed in any
factory by this Act or the rules made thereunder, there shall be displayed in every factory a notice
containing such abstracts of this Act and of the rules made thereunder, in English and in the ver
nacular of the majority of the workers as the 1[Provincial Government] may prescribe.
(2) All notices required to be displayed in a factory shall be displayed at some conspicuous place
at or near the main entrance to the factory, and shall be maintained in a clean and legible condition.
77. Power of Provincial Government to make rules. The 2[Provincial Government] may make
rules3 requiring occupiers or managers of factories to submit such returns occasional or periodical, as
may in 4[its] opinion be required for the purposes of this Act.
78. [Control of rules made by L. Gs.]—Rep. by A.O., 1937.
79. Publication of rules.__ (1) All rules made under this Act shall be subject to the condition of
previous publication, and the date to be specified under clause (3) of section 23 of the General
Clauses Act, 1897 (X of 1897), shall not be less than three months from the date on which the draft
of the proposed rules was published.
(2) All such rules shall be published in 5* * * the 6[official Gazette] 7* * * and shall,
unless some later date is appointed, come into force on the date of such publication.
80. Application to Government factories. This Act shall apply to factories belonging to the
8[Government].
81. Protection to persons acting under this Act. No suit, prosecution or other legal proceeding
shall lie against any person for anything which is in good faith done or intended to be done under this
Act.
82. [Repeal and Savings.] Rep. by the Repealing and Amending Act, 1937 (XX of 1937) s.3 and
2nd Sch.
THE SCHEDULE.__Rep. by Act XX of 1937, s.3 and Sch.II.
_______
1Subs. by A. O. 1937, for “L.G”.
2Subs. ibid, for “G.G. in C”.
3For the Karachi Factories (Returns) Rules, see Gazette of Karachi 1960, Pt. II, pp 246256.
4Subs. by A.O., 1937 for “his”.
5The words “The Gazette of India or” omitted by A. O., 1937.
6Subs. ibid, for “local official Gazette”.
7The words “as the case may be,” omitted ibid.
8Subs. by A. O. 1961, Art. 2, for “Crown” (with effect from the 23rd March 1956).
Page 48 of 49](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-308-320.jpg)




![TEXT
1
THE 2
[PUNJAB] SHOPS AND ESTABLISHMENTS ORDINANCE, 1969
(VIII of 1969)
[3rd June, 1969]
An
Ordinance
to amend and consolidate the law relating to the hours and other conditions of work
and employment of persons employed in shops and commercial, industrial and other
establishments in 3
[the Punjab] and matters connected therewith.
Preamble.– WHEREAS it is expedient to amend and consolidate the law relating to
the hours and other conditions of work and employment of persons employed in
shops and commercial, industrial and other establishments
4
[in the Punjab] and
matters connected therewith;
NOW, THEREFORE, in pursuance of the Martial Law Proclamation of 25th
March, 1969, read with the Provisional Constitution Order, the Administrator of
Martial Law, Zone A, in exercise of the powers of the Governor of West Pakistan
conferred on him by the Chief Martial Law Administrator, is pleased to make and
promulgate the following Ordinance:–
1. Short title, extent, commencement and application.– (1) This Ordinance
may be called the 5
[Punjab] Shops and Establishments Ordinance, 1969.
(2) It extends to the whole of 6
[the Punjab].
(3) It shall come into force at once in such areas, and its provisions shall
automatically apply to such establishments or classes thereof, to which any law on
the subject was applicable immediately before the coming into force of this
Ordinance.
(4) Government may, by notification in the official Gazette, extend the
operation of this Ordinance or any provisions thereof to any other area or
establishment, or exclude any area or establishment to which it extends, from its
operation.
1
This Ordinance was promulgated by the Administrator Martial Law, Zone ‘A’ on 30th June, 1969; published in the West
Pakistan Gazette (Extraordinary), dated 30th July, 1969, pages 1057-1076; saved by Article 281 of the Interim Constitution of
Pakistan (1972); and, validated by the Validation of Laws Act, 1975 (LXIII of 1975).
2
Substituted for the words “West Pakistan” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.2; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
3
Substituted for the words “West Pakistan” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.2; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
4
Substituted for the words “in West Pakistan” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.2; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
5
Substituted for the words “West Pakistan” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.2; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
6
Substituted for the words “Pakistan” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014,
s.2; and published in the Punjab Gazette (Extraordinary), pages 2647-2648. Which were earlier substitutedfor the words and
comma“West Pakistan, except the Tribal Areas” by the Federal Adaptation of Laws Order, 1975 (P.O. No.4 of 1975),
w.e.f.1.8.1975, Article 2 and the Schedule; and published in the Gazette of Pakistan (Extraordinary), pages 435-467.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-313-320.jpg)
![2. Definitions.– In this Ordinance, unless the context otherwise requires–
7[(a) “adolescent” means a person who has attained the age of fifteen years
but has not attained the age of eighteen years;]
8[(aa) “adult” means a person who has completed his eighteenth year of age;]
(b) “apprentice” means a person who is employed, whether on payment of
wages or not, for the purposes of being trained in any trade, craft or
employment in any establishment;
9[(bb) “Authority” means the Authority appointed under section 15 of the
Payment of Wages Act, 1936 (IV of 1936);]
(c) “child” means a person who has not completed his 10
[11
[fifteenth]] year of
age;
(d) “closed” means not open for the service of any customer or to any
business connected with the establishment;
(e) “commercial establishment” means an establishment which carries on
any business, trade or profession or any work in connection with, or
incidental or ancillary to, any business, trade or profession, and
includes–
(i) a society registered under the Societies Registration Act, 1860
(XXI of 1860), and a charitable or other trust, whether registered
or not, which carries on, whether for purposes of gain or not, any
business, trade or profession, or any work in connection with or
incidental or ancillary thereto;
(ii) an establishment wherein there is conducted the business of
advertising, commission, forwarding or a commercial agency;
(iii) a clerical department 12[*****] of any industrial or commercial
undertaking;
13[iv) an insurance company, joint stock company, Information
Technology (IT) company, software house, bank, brokers’ offices
or exchange and office of lawyers, income-tax practitioners, IT
7
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
8
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
9
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
10
Substituted for the word “twelfth” by the West Pakistan Shops and Establishments (Punjab Amendment) Act, 1975 (X of 1975),
w.e.f.5.3.1975, s.2; and published in the Punjab Gazette (Extraordinary), pages 147-YY to 147-ZZ, and the same substituted by
the Labour Laws (Amendment) Act, 1977 (XVII of 1977), w.e.f.9.5.1977, s.2 and the first Schedule; and published in the Gazette
of Pakistan (Extraordinary), pages 339-344 .
11
Substituted for the word “fourteenth” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published
in the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
12
Omitted the words “of a factory or” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in
the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
13
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-314-320.jpg)
![professionals, registered accountants, contractors, engineers
and private clinics of doctors;]
(v) such other professional establishment or class thereof as
Government may, by notification in the official Gazette, declare
to be commercial establishment for the purposes of this
Ordinance;
but does not include a factory, shop, residential hotel, restaurant,
eating house, theatre or other place of public amusement or
entertainment;
(f) “day” means the period of twenty four hours beginning at mid-night,
provided that in the case of an employee, whose hours of work extend
beyond mid-night, day means the period of twenty four hours beginning
when such employment commences, irrespective of mid-night;
(g) “employee” means any person employed whether directly or otherwise,
about the business of an establishment for the owner or occupier
thereof, even though he receives no reward or remuneration for his
labour, but does not include a member of the employer’s family;
(h) “employer” means a person owning or having charge of the business of
an establishment, and includes an agent or manager or any other
person acting on behalf of such person in the general management or
control of such establishment;
(i) “employer’s family” means the employer’s husband or wife, as the case
may be, sons, daughters, father, mother, and brothers and sisters living
with and dependent on the employer;
14[(j) “establishment” means a shop, commercial establishment, industrial
establishment, private healthcare facilities, residential hotel, restaurant,
eating house, cafe, cinema, theatre, circus, or other place of public
amusement or entertainment, schools, educational institutions, teaching
academies, technical and vocational training institutes and such other
establishments or class thereof as Government may, by notification in
the official Gazette, declare to be establishments for the purposes of
this Ordinance;]
(k) “factory” means a factory as defined in clause (j) of section 2 of the
Factories Act, 1934 (XXV of 1934);
(l) “form” means a form specified in the Schedule;
15
[(m) “Government” means Government of the Punjab;]
(n) “hours of work” or “working hours” with reference to an establishment
means the time during which the employees in the establishment are at
14
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
15
Substituted by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014, s.3; and published in the
Punjab Gazette (Extraordinary), pages 2647-2648.which were earlier Substituted for the words “ Government of West Pakistan ”
by the Federal Adaptation of Laws Order, 1975 (P.O. No.4 of 1975), w.e.f.1.8.1975, Article 2 and the Schedule; and published in
the Gazette of Pakistan (Extraordinary), pages 435-467.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-315-320.jpg)
![the disposal of the employer, exclusive of any interval allowed for rest
and meals;
(o) “industrial establishment” means a workshop or other establishment in
which the work of 16[mending, assembling, fabricating, blending,
construction of building, erection of machinery,] making, altering,
repairing, ornamenting, finishing or packing or otherwise treating any
article or substance with a view to its use, sale, transport, delivery, or
disposal is carried on, or where any such service is rendered to a
customer, and includes such other class or classes of establishments
as Government may, by notification in the official Gazette, declare to be
industrial establishments for the purposes of this Ordinance, but does
not include a factory;
(p) “permanent employee” means an employee who has been engaged on
a permanent basis, and includes an employee who has completed nine
months continuous service in one or different occupations in the same
establishment, including breaks due to sickness, accident, leave, illegal
lock outs, legal strikes or involuntary closure of the establishment, and
has satisfactorily completed a probationary period of three months;
(q) “prescribed” means prescribed by rules made under this Ordinance;
(r) “residential hotel” means any premises in which a bona fide business is
carried on for the supply of dwelling accommodation and meals on
payment of a sum of money by a traveller or any other member of the
public or class of the public and includes a club;
(s) “restaurant” and “eating house” mean any premises in which is carried
on wholly or principally the business of the supply of meals or
refreshments to the public or a class of the public for consumption on
the premises;
(t) “retail trade” includes the business of a barber or hair-dresser, the sale
of refreshments or intoxicating liquors, and sales by auctions;
(u) “shop” means any premises used wholly or in part for the whole-sale or
retail sale of commodities or articles, either for cash or on credit, or
where services are rendered to customers, and includes an office, a
store room, godown, warehouse or place of work, whether in the same
premises or otherwise, mainly used in connection with such trade or
business;
(v) “temporary employee” means an employee who has been engaged for
work which is of an essentially temporary nature likely to be finished
within a period not exceeding nine months;
(w) “wages” means wages as defined in the Payment of Wages Act, 1936
(IV of 1936);
16
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-316-320.jpg)
![(x) “week” means a period of seven days beginning at mid-night on
Saturday night17[.]
18[* * * * * * * * * * * *]
3. References to time of day.– References to time of day in this Ordinance are
references to 19
[Pakistan] Standard time.
4. Power to grant exemptions.– Government may, by notification in the official
Gazette, exempt from the operation of all or any of the provisions of this Ordinance
any establishment or any class thereof or any employer or employee or class of
employers or employees on such conditions as it may think fit.
5. Ordinance not applicable to certain establishments and persons.– (1)
Nothing in this Ordinance shall apply to–
20
[(i) Offices of the Government 21[as mentioned in the Punjab Government
Rules of Business] or the Federal Government;]
(ii) offices of or under the Pakistan
22
[*] Railway Board, including railway
stations;
(iii) offices of or under any 23[local government,] local authority, a trust, a
corporation or any other public statutory body, which is not run for profit
or gain or in the course of its business does not make any profit or gain;
(iv) shops or stalls in any public exhibition or show, in so far as such shops
or stalls deal in retail trade which is solely subsidiary or ancillary to the
main purpose of such exhibition or show;
(v) shops or stalls in any public fair or bazar held for religious or charitable
purposes;
(vi) clubs, hostels and messes not maintained for profit or gain;
(vii) establishments for the treatment or care of the sick, infirm, destitute or
mentally unfit persons 24[financed and managed by the Government,
Federal Government, local government or any local authority of the
Government];
17
Substituted for the “semi colon” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
18
Omitted the “clause y” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab
Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.2.
19
Substituted for the words “West Pakistan” by the Federal Adaptation of Laws Order, 1975 (P.O. No.4 of 1975), w.e.f.1.8.1975,
Article 2 and the Schedule; and published in the Gazette of Pakistan (Extraordinary), pages 435-467.
20
Substituted by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014, s.4; and published in the
Punjab Gazette (Extraordinary), pages 2647-2648.
21
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.3.
22
Omitted the words “Western”by the Federal Adaptation of Laws Order, 1975 (P.O. No.4 of 1975), w.e.f.1.8.1975, Article 2 and
the Schedule; and published in the Gazette of Pakistan (Extraordinary), pages 435-467.
23
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.3.
24
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.3.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-317-320.jpg)
![(viii) stalls and refreshment rooms at railway stations, steamer and launch
stations, docks, wharves and air ports, and on trains, steamers or air
crafts, so far as the sale of commodities is concerned;
(ix) any person employed as manager, travelling agent, canvasser,
messenger, watchman, caretaker or conservancy staff or any person
employed exclusively in connection with the collection, despatch,
delivery, and conveyance of, or custom formalities on, goods;
(x) any person employed for the business of any shop or commercial
establishment mentioned in clauses (i) to (viii).
(2) Nothing 25[*****] in section 7 shall apply to–
(i) clubs, hostels and messes maintained for profit or gain, so far as
service and attendance upon customers is concerned;
(ii) shops dealing solely in any vegetables, meat, fish, dairy products,
bread, pastries, sweet-meats and flowers, so far as the sale of these
articles is concerned;
(iii) shops dealing mainly in medicines, surgical appliances, bandages or
other medical requisites, so far as the sale of these articles is
concerned;
(iv) shops dealing in articles required for funerals, burials or cremations, so
far as the sale of these articles is concerned;
(v) shops dealing mainly in tobacco, cigars, cigarettes, biries, pan, liquid
refreshments sold retail for consumption on the premises, ice,
newspapers or periodicals, so far as the sale of these articles is
concerned;
(vi) automobile service stations (not being repair shops) and petrol pumps
for the retail sale of petrol;
(vii) barbers and hair-dressers’ shops, so far as service to customers is
concerned;
(viii) cinemas, theatres and other places of public entertainment.
(3) Notwithstanding anything contained in sub-section (2), Government
may, by general or special order, fix the opening and closing hours for all or any of
the classes of establishments specified therein.
(4) Notwithstanding anything contained in sub-sections (1) and (2),
Government may, by notification in the official Gazette, direct that any of the
establishments or persons specified therein shall not be exempted from the operation
of such provisions of this Ordinance as are specified in such notification, and
thereupon the provisions of this Ordinance specified in such notification shall apply to
such establishments or persons.
6. Weekly holiday in establishments.–
26
[(1) Except as otherwise provided in
this Ordinance, every person employed in any establishment shall, in addition to the
25
Omitted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.3.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-318-320.jpg)
![leave and holidays as may be admissible to him under sections 14, 15 and 16, be
allowed as holiday, one day in each week.]
(2) No deduction on account of any holiday allowed under sub-section (1)
shall be made from the wages of any employee of any establishment.
(3) If an employee is employed on daily wages, he shall none-the-less be
paid his daily wages for the holiday, and where an employee is paid on piece-rate, he
shall receive for the holiday the average of the wages received during the week.
27
[(4) * * * * * * * * * * * ]
(5) The choice of a closed day shall rest with the employer, who shall
intimate such choice to the 28[Inspector appointed under section 25 of this Ordinance:]
(a) in the case of an establishment existing at the time this Ordinance
comes into force, within two months thereof; and
(b) in the case of an establishment set up after the coming into force of this
Ordinance, or to which the provisions of this Ordinance are
subsequently applied, within two months of the setting up of the
establishment or the application of the provisions of this section thereto,
as the case may be.
(6) An employer who has intimated his choice of a closed day under the
provisions of sub-section (5), shall not change the closed day for the establishment
without the prior approval in writing of the 29[Inspector].
30
[7. Opening and closing hours of establishments.– (1) No establishment shall
on any day remain open after 8:00 p.m.:
Provided that any customer who was being or was waiting in the establishment
to be served at such hour, may be served during the period of thirty minutes
immediately following such hour:
Provided further that Government may, by notification in the official Gazette, fix
any other hour after 31[or before] which establishments generally or any class of
establishment shall not remain open.
(2) Every employer shall display, at a prominent place in the establishment,
a board specifying the hours during which the establishment will remain open.
(3) No employee shall be required or permitted to work continuously in any
establishment for more than six hours in the case of an adult and for more than three
26
Substituted by the Finance Act, 2006 (III of 2006), w.e.f.1.7.2006, s.9; and published in the Gazette of Pakistan
(Extraordinary), pages 183-567.
27
Omitted by the Finance Act, 2006 (III of 2006), w.e.f.1.7.2006, s.9; and published in the Gazette of Pakistan(Extraordinary),
pages 183-567.
28
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.4.
29
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.4.
30
Substituted by the West Pakistan Shops and Establishment (Amendment) Ordinance, 1969 (XVII of 1969), w.e.f.16.8.1969,
s.2; and published in the Gazette of West Pakistan (Extraordinary), pages 1325-A to 1325-B.
31
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.5.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-319-320.jpg)
![32[*****] hours in the case of a 33[an adolescent], unless he has been allowed an
interval for rest or meal of not less than one hour.
(4) Except with the permission of Government, no woman 34[*****] shall be
employed in any establishment otherwise than between the hours of 9:00 a.m. to
35[8]:00 p.m.]
36[8. Daily, weekly hours and overtime.– (1) Save as otherwise expressly
provided in this Ordinance, no adult employee shall be required or permitted to work
in any establishment in excess of nine hours a day and forty-eight hours a week, and
no adolescent in excess of seven hours a day and forty-two hours a week:
Provided that in any day or in any week, in which there occurs stock-taking,
making up of accounts, settlement or such other business operation, and during such
other periods as may be prescribed, an adult employee of an establishment may be
required or permitted to work over-time in such establishment for more than nine
hours in such day and for more than forty-eight hours in such week, but so that the
total number of hours so worked by an adult does not exceed six hundred and twenty
four hours in any one year.
(2) The employer shall not:
(a) allow an adolescent to work in an establishment except
between 9:00 a.m. and 8:00 p.m.;
(b) allow an adolescent to work in any establishment on any day
on which he has already been working in another
establishment;
(c) fix the hours of an adolescent in such manner that the working
hours are in conflict with the timings of the educational or
vocational institution, where he is enrolled; and
(d) allow an adolescent to work overtime.]
9. Over-time wages.– When any employee is required to work over-time in any
establishment, as provided in the proviso to section 8, the wages payable to such
employee in respect of such over-time work shall be calculated at double the ordinary
rate of wages payable to him37
[:]
38
[Provided that no overtime shall be payable to the contract worker employed
on piece rate basis.]
32
Omitted the words “and half” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.5.
33
Substituted for the words “young person” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023),
published in the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.5.
34
Omitted the words “or young persons” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023),
published in the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.5.
35
Substituted for the figure “7” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.5.
36
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.6.
37
Substituted for the full-stop by the Finance Act, 2006 (III of 2006), w.e.f.1.7.2006, s.9; and published in the Gazette of Pakistan
(Extraordinary), pages 183-567.
38
Added by the Finance Act, 2006 (III of 2006), w.e.f.1.7.2006, s.9; and published in the Gazette of Pakistan (Extraordinary),
pages 183-567.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-320-320.jpg)
![39[10. Spread-over.– The period of work of an adult and adolescent shall be so
arranged that inclusive of the interval for rest or meals under section 7, it shall not
spread-over more than twelve hours in the case of adult and seven hours in the case
of adolescent:
Provided that the total period of work so worked out, in case of an adult
employee, shall not exceed sixty hours and by an adolescent forty-two hours in a
week.]
40
[10-A. Daycare rooms for children.– (1) Where twenty-five or more women are
employed in an establishment, the employer shall reserve a suitable daycare room
for under-six years old children of the women.
(2) The daycare room shall be established, managed and conformed to
such standards, as may be prescribed.]
11. Time and conditions of payment of wages.– (1) Every employer or his
agent or the manager of an establishment shall fix the period in respect of which
wages to employees shall be payable and shall be responsible for the payment to
persons employed by him of all wages required to be paid under this Ordinance.
(2) No wage period, so fixed, shall exceed one month.
(3) The wages of every employee in any establishment shall be paid on a
working day before the expiry of the seventh day of the last day of the wage period in
respect of which the wages are payable.
41[(4) All wages shall be paid in legal tender in such manner as may be
prescribed.]
(5) Where the employment of any person is terminated by or on behalf of
the employer, the wages and other dues earned by such person shall be paid before
the expiry of the second working day after the day on which his employment is
terminated.
12. Claims arising out of delay in payment of wages and penalty for
malicious or vexatious claims.– 42[(1) The Authority shall hear and decide all
claims arising out of delay in the payment or non-payment of the wages of employees
in the area assigned to it.]
(2) When contrary to the provisions of this Ordinance, wages of any
employee have been delayed or withheld, such employee himself or through any
39
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.7.
40
Inserted by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014, s.5; and published in the
Punjab Gazette (Extraordinary), pages 2647-2648.
41
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.8.
42
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-321-320.jpg)
![other person, whom he may authorise in this behalf, may within 43
[one year] from the
day on which such payment was to be made, apply to the Authority 44[*****]:
Provided that an application may be admitted after the said period of 45
[one
year] but not later than 46
[one year and six months], if the applicant satisfies the
Authority that he had sufficient cause for not making the application within such
period.
(3) When any application under sub-section (2) is entertained, the Authority
shall hear the applicant and the employer or other person responsible for the
payment of wages or give them an opportunity of being heard and, after such further
inquiry, if any, as may be necessary, may without prejudice to any other penalty to
which such employer or other person is liable under this Ordinance, direct that
payment be made to the applicant of delayed wages together with the payment of
such penalty, not exceeding 47
[two thousand] rupees, as the Authority may fix:
Provided that no direction for the payment of penalty shall be made in the case
of delayed wages if the Authority is satisfied that the delay was due to–
(a) a bona fide error or bona fide dispute as to the amount payable to the
employee; or
(b) the occurrence of an emergency, or the existence of such exceptional
circumstances that the person responsible for the payment of the
wages was unable to make prompt payment; or
(c) the fault of the employee.
(4) If the Authority hearing any application under this section is satisfied
that 48[the application] was either malicious or vexatious, the Authority may direct that
a penalty not exceeding 49[five hundred] rupees be paid to the employer or other
person responsible for the payment of wages by the person presenting the
application.
(5) Any amount directed to be paid under this section may be recovered–
(a) if the Authority is a Magistrate, by the Authority as if it were a fine
imposed by him as Magistrate; and
50[(b) if the Authority is not a Magistrate, by the Authority as arrears of land
revenue or, in the prescribed manner by distress and sale of the
43
Substituted for the words “four months” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.6; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
44
Omitted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
45
Substituted for the words “four months” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.6; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
46
Substituted for the words “six months” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.6; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
47
Substituted for the words “fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014, s.6;
and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
48
Substituted the word “it” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab
Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
49
Substituted the word “fifty” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
50
Substituted for the word “fifty” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-322-320.jpg)
![moveable property belonging to the person by whom the amount is to
be paid, or by the attachment and sale of the immovable property
belonging to such person.]
(6) An appeal against a direction made by the Authority under sub-section
(3) or sub-section (4) may be referred to the 51
[Labour Court establishment under the
Punjab Industrial Relations Act, 2010 (XIX of 2010)] within thirty days of the date on
which the direction was made–
(a) by the employer or other person responsible for the payment of wages
under section 11 if the total sum directed to be paid by way of wages
and penalty exceeds 52[two] 53
[thousand] rupees; or
(b) by an employee, if the total amount of wages claimed to have been
withheld from him or from the unpaid group to which he belonged,
exceeds 54[one hundred] rupees; or
(c) by any person directed to pay a penalty under sub-section (4).
(7) If there is no appeal, the direction of the Authority made under sub-
section (3) or sub-section (4) shall be final, and where there is an appeal as provided
in sub-section (6), the decision in appeal shall be final.
(8) 55[The Authority] shall, for the purposes of determining any matter
referred to in sub-section (3) or sub-section (4)–
(a) have all the powers as are vested in a Civil Court under the Code of
Civil Procedure, 1908 (V of 1908), for enforcing the attendance of
witnesses, compelling the production of documents , and taking of
evidence; and
(b) be deemed to a Civil Court for all the purposes of section 195 and
Chapter XXXV of the Code of Criminal Procedure, 1898 (V of 1898).
13. Bar of suits.– No Court shall entertain any suit for the recovery of wages in so
far as the sum so claimed–
(a) forms the subject of an application made under sub-section (2) of
section 12, which is pending before the Authority 56[*****], or of an
appeal under sub-section (6) of the said section;
(b) has formed the subject of a direction made under sub-section (3) of
section 12;
51
Substituted for the words “District Court” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.6; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
52
Substituted for the word “one” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
53
Substituted for the words “hundred” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014,
s.6; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
54
Substituted for the word “fifty” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
55
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.9.
56
Omitted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.10.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-323-320.jpg)
![(c) has been adjudged in any proceedings under section 12 not to be owed
to an employee; or
(d) could have been recovered by an application under section 12.
14. Annual leave.– (1) Every employee shall be allowed leave with full wages for
a period of fourteen days after continuous employment in an establishment, whether
in the same or different capacities, for a period of twelve months.
(2) If an employee does not in any period of twelve months avail of the
whole or any part of the leave allowed to him under sub-section (1)–
(a) any leave not availed of by him shall be added to the leave to be
allowed to him under that sub-section in the succeeding period of
twelve months; provided that when the total leave due to an employee
under this section amounts to thirty days, no further accumulation of or
addition to such leave will be permissible;
(b) he may, at his request, in lieu of the leave not availed of by him, be paid
by the employer full wages for such leave.
(3) For the purposes of computing the period during which an employee
has been in continuous employment within the meaning of sub-section (1), the period
during which he was on leave under this section, or sections 15 and 16, shall be
included.
15. Casual and sick leave.– (1) Every employee shall be entitled to casual leave
with full wages for ten days in a calendar year. Such leave shall not ordinarily be
granted for more than three days at a time and shall not be accumulated.
(2) Every employee shall be entitled to sick leave with full wages for a total
period of eight days in every year. Such leave, if not availed of by any employee
during a calendar year, may be carried forward, but the total accumulation of such
leave shall not exceed sixteen days at any one time.
16. Festival holidays.– Every employee shall be allowed ten days festival
holidays with full wages in a year. The days and dates for such festival holidays shall
be notified to the employees by the employer in the beginning of the calendar year.
17. Wages during leave or holiday period.– (1) For each day of the leave or
holidays allowed to an employee under sections 14, 15 and 16, he shall be paid at
the rate equivalent to the daily average amount, which, during the three months
preceding the leave or holidays, was being earned by the employee.
(2) An employee, who has been allowed leave under section 14 for any
period not less than four days in the case of an adult and five days in the case of 57[an
adolescent], shall before the leave begins, be paid his wages for the period of the
leave allowed.
18. Sections 14, 15, 16 and 17 not to apply to certain establishments.– The
provisions of sections 14, 15, 16 and 17 shall not apply in relation to employees
employed in commercial establishments as defined in clause (b) of section 2 of the
57
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.11.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-324-320.jpg)
![West Pakistan Industrial and Commercial Employment (Standing Orders) Ordinance,
1968 (West Pakistan Ordinance VI of 1968).
19. Termination of employment.– (1) For terminating employment of a
permanent employee, one month’s notice in writing shall be given either by the
employer or by the employee and in lieu of notice, one month’s wages calculated on
the basis of average of wages earned during the preceding three months shall be
paid.
(2) No temporary employee, whether monthly rated, weekly rated or daily
rated, and no apprentice shall be entitled to any notice or pay in lieu thereof if his
services are terminated, but the services of a temporary employee shall not be
terminated as a punishment unless he has been given an opportunity of explaining
the charges levelled against him.
20. Prohibition of employment of children.– No child shall be required or
allowed to work in any establishment.
58[20-A. Adolescent to carry card giving reference to certificate of age.– No
adolescent shall be allowed to work in any establishment unless:
(a) a certificate of his registration with the National Database and
Registration Authority (NADRA) or a certificate of his birth registration
with the respective local government are in the custody of the employer;
and
(b) he carries, while at work, the card issued by the employer giving a
reference to such certificate.]
59[20-B. Register of adolescents on work.– The employer of every
establishment shall maintain a register containing the following particulars of the
adolescents working in his establishment:
(a) name and date of birth of each adolescent in employment of the
establishment;
(b) date of start of employment of the adolescent;
(c) nature of work assigned to the adolescent and his working hours, which
shall not be in conflict with the provisions of the Punjab Restriction on
Employment of Children Act 2016 (L of 2016);
(d) reference number of certificate mentioned in section 20-A and its date
of issuance;
(e) blood group of the adolescent;
(f) contact number of the adolescent and his near relative for contact in
case of an emergency; and
(g) such other particulars of the adolescent as may be prescribed.]
21. Contracting out.– Any contract or agreement, whether made before or after
the commencement of this Ordinance, whereby an employee relinquished any right
58
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.12.
59
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.12.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-325-320.jpg)
![conferred by this Ordinance shall be null and void in so far as it purports to deprive
him of such right.
60[21-A. Compulsory vaccination and inoculation.– (1) Every employee in an
establishment shall be vaccinated and inoculated against such diseases and at such
intervals as may be prescribed.
(2) The expenses of compulsory vaccination and inoculation as mentioned
at sub-section (1) shall be borne by the employer of the establishment.]
61[21-B. Provision of canteen.– (1) The Government may direct that in an
establishment wherein more than one hundred employees are ordinarily employed,
an adequate canteen shall be provided for the employees in such manner as may be
prescribed.
(2) Without prejudice to the generality of sub-section (1), the rules under
that sub-section may provide for:
(a) the date by which such canteen shall be made functional;
(b) the standards of construction, accommodation, furniture and
other necessities of the canteen;
(c) the foodstuff to be served therein and the charges thereof; and
(d) representation of the employees in the management of canteen.]
62[21-C. Prohibition on lifting excessive weight.– (1) No person shall be
employed in any establishment to lift, carry or move any load so heavy as to likely
cause him an injury.
(2) The Government may make rules prescribing the maximum weights
which may be lifted, carried or moved by an adult man, adult woman and adolescent
employed in an establishment or in any class or description of establishments or in
carrying on any specified process.]
63[21-D. Provision of separate toilets.– The employer shall make provision of
enclosed and separate toilets for male and female employees, and such toilets shall
be maintained in a clean and sanitary condition at all times.]
64[22. Guarding of machinery.– In every industrial establishment, all mechanically
or electrically propelled machinery shall be properly guarded by the employer to avoid
any occupational hazard or injury to the employee in such manner as may be
prescribed.]
60
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.13.
61
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.13.
62
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.13.
63
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.13.
64
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.14.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-326-320.jpg)
![23. Maintenance of records and registers.– Every employer shall, for the
purpose of this Ordinance, maintain such records and registers and furnish such
information 65[to the Authority on demand] as may be prescribed.
24. Registration of establishment 66
[*****].– (1) Every establishment, other than
a one man shop, as hereinafter defined, and factories employing clerical staff within
the factory premises, shall be registered with the Deputy Chief Inspector for the area
within which such establishment is situated.
67[68[(2)An application for registration of an establishment shall be made by the
employer as per format in Form ‘A’.
Explanation– For the purposes of this section, “one man shop” means a shop
run by an employer or by any member of employer’s family without engaging an
employee.]
(3) An application for registration of an establishment shall be made–
(a) in the case of an establishment existing at the time this Ordinance
comes into force, within three months thereof; and
(b) in the case of an establishment set up after the coming into force of this
Ordinance or to which the provisions of this Ordinance are
subsequently applied, within two months of the setting up of the
establishment or the application of this Ordinance thereto, as the case
may be.
(4) On receipt of the application 69[*****] specified in sub-section (2), the
Deputy Chief Inspector shall, on being satisfied about the correctness of the
application, register the establishment in the Register of Establishments to be
maintained in form ‘B’ and shall issue a registration certificate to the employer in form
‘C’70[:]
71[Provided that such registration and issuance of registration certificate may
be done through electronic mode online.]
(5) The registration certificate shall be prominently displayed by the
employer at the establishment and shall be renewed after every two years 72[or such
other interval as the Government may notify in the official Gazette].
65
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.15.
66
Omitted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.
67
Substituted by the Punjab Shops and Establishments (Amendment) Ordinance 2018 (VI of 2018), published in the Punjab
Gazette (Extraordinary), dated: 01 June 2018, pp,8415-8416 s.2
68
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.
69
Omitted the words “and the fees” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in
the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.
70
Substituted for the “full stop” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.
71
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.
72
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.16.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-327-320.jpg)
![25. Appointment of Chief Inspector, Deputy Chief Inspectors and
Inspectors.– (1) Government may, by notification in the official Gazette, appoint–
(a) a Chief Inspector of Shops for the whole of the Province;
(b) Deputy Chief Inspectors of Shops for such areas as may be notified;
and
(c) such persons or class of persons as it thinks fit to be Inspectors for the
purposes of this Ordinance within such local limits as may be specified
by the Chief Inspector of Shops.
(2) The Chief Inspector of Shops and the Deputy Chief Inspectors of
Shops–
(a) shall supervise the work of Inspectors appointed under clause (c) of
sub-section (1) in such manner as may be prescribed; and
(b) may exercise all or any of the powers of an Inspector.
(3) The Chief Inspector of Shops, Deputy Chief Inspectors of Shops and
Inspectors appointed under sub-section (1) shall be deemed to be public servants
within the meaning of section 21 of the Pakistan Penal Code (XLV of 1860).
26. Powers of Inspectors.– An Inspector appointed under section 25 may, for the
purposes of this Ordinance and within the local limits for which he is appointed, at all
reasonable times enter into any place which is, or which he has reason to believe is,
an establishment, with such assistants, if any, being persons in the service of
Government, and make such examination of that place or of any prescribed record,
register, or other documents maintained therein, and may require such explanation of
any prescribed record, register or other documents and do all such things as he
considers necessary for the purpose of this Ordinance.
27. Penalties.– (1) If any employer, with intent to deceive, makes or causes or
allows to be made, in any register, record or notice required to be maintained under
the provisions of this Ordinance or the rules made thereunder, any entry, or wilfully
omits or causes or allows to be omitted from any such register, record or notice, any
entry which is required to be made thereunder, or maintains or causes or allows to be
maintained more than one set of any such register, record or notice except the office
copy of such notice, or sends or causes or allows to be sent to an Inspector any
statement, information or notice required to be sent under the provisions of this
Ordinance or the rules made thereunder, which to his knowledge is false in any
material particulars, he shall, on conviction, be punished with fine which shall not be
less than 73
[74[two thousand]] rupees and which may extend to 75
[five thousand]
rupees.
(2) Whoever contravenes any of the provisions of section 6, 7, 19 or 20
shall, on conviction, be punishable with fine which for the first offence may extend to
73
Substituted for the word “fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014), w.e.f.24.2.2014, s.8;
and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
74
Substituted for the words “one thousand” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023),
published in the Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.17.
75
Substituted for the words “two hundred and fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.8; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-328-320.jpg)
![rupees 76
[five thousand], and for a second or subsequent offence with fine which may
extend to rupees 77
[ten thousand] or with simple imprisonment which may extend to
three months, or with both.
(3) Whoever contravenes any other provisions of this Ordinance shall, on
conviction, be punishable with fine which for the first offence may extend to rupees
78
[three thousand], and for a second or any subsequent offence, to rupees 79
[five
thousand] or with simple imprisonment which may extend to three months, or with
both.
28. Procedure.– (1) No prosecution under this Ordinance or any rules made
thereunder shall be instituted except by or with the previous sanction of an Inspector,
or other officer or authority specially empowered by Government in this behalf.
(2) No Court inferior to that of a Magistrate of the first class shall try an
offence punishable under this Ordinance or any rule made thereunder.
80[(3) Every offence under section 27 shall be cognizable on the complaint
made by an Inspector appointed under this Ordinance, and may be tried summarily.]
81[28-A. Presumption as to employment.– If a child over the age of six years is
found inside any part of an establishment in which adolescents are employed, he
shall, until the contrary is proved, be deemed to be working in the establishment.]
29. Limitation of prosecutions.– No Court shall take cognizance of any offence
punishable under this Ordinance or any rule made thereunder unless complaint
thereof is made within three months from the date on which the alleged commission
of the offence comes to the knowledge of an Inspector.
30. Indemnity.– No suit, prosecution or legal proceedings shall lie against any
person in respect of anything done in good faith under this Ordinance or the rules
thereunder.
31. Delegation of powers.– Government may, by notification in the official
Gazette, delegate all or any of its powers under this Ordinance or the rules
thereunder to any subordinate authority 82[,the concerned Secretary to the
Government] or agency as may be considered expedient by it.
32. Power to make rules.– (1) Government may, by notification in the official
Gazette, make rules for carrying out the purposes of this Ordinance.
76
Substituted for the words “two hundred and fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.8; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
77
Substituted for the words “five hundred” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.8; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
78
Substituted for the words “one hundred and fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.8; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
79
Substituted for the words “two hundred and fifty” by the Punjab Shops Establishments (Amendment) Act 2014 (II of 2014),
w.e.f.24.2.2014, s.8; and published in the Punjab Gazette (Extraordinary), pages 2647-2648.
80
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.18.
81
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.19.
82
Inserted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.20.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-329-320.jpg)
![(2) In making rules under this section, Government may direct that any
person committing breach thereof shall, on conviction, be punishable with fine which
may extend to 83[one thousand] rupees, and where the breach is a continuing one,
with a further fine which may extend to 84[fifty] rupees for every day, after the first,
during which the breach continues.
33. Saving of certain rights and privileges.– Nothing in this Ordinance shall
affect any right or privilege to which an employee is entitled on the date of the
commencement of this Ordinance under any law for the time being in force or under
any award, agreement, settlement, contract, custom or usage which is in force on
that date, if such right or privilege is more favourable to him than any right or privilege
conferred upon him by this Ordinance.
34. Repeal and saving.– (1) The following enactments, hereinafter referred to as
the said Acts, are hereby repealed:–
(a) The Sindh Shops and Establishments Act, 1940 ( Sind XVIII of 1940);
(b) The Punjab Trade Employees Act, 1940 (Punjab X of 1940);
(c) The North-West Frontier Trade Employees Act, 1947 (N.W.F.P. XX of
1947); and
(d) The Weekly Holidays Act, 1942 (XVIII of 1942).
(2) Notwithstanding the repeal of the said Acts, everything done, orders
passed, action taken, obligation, liability, penalty or punishment incurred, enquiry or
proceeding commenced, officer appointed or person authorised, jurisdiction or power
conferred, rule made or notification issued, under any of the provisions of the said
Acts, shall, if not inconsistent with the provisions of this Ordinance, continue in force
and be deemed to have been done, passed, taken, incurred, commenced, appointed,
authorised, conferred, made or issued under the provisions of this Ordinance.
83
Substituted for the word “fifty” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.21.
84
Substituted for the word “ten” by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the
Punjab Gazette (Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.21.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-330-320.jpg)
![SCHEDULE
[See SECTION (2) (l) AND SECTION 24]
85[FORM ‘A’
APPLICATION FORM
1. Name of the establishment, if any.
2. Complete Postal address of the establishment including name of city,
tehsil and the district.
3. E-mail and web address of the establishment.
4. GPS coordinates of the location of the establishment.
5. Full name of the employer (including his father’s/ husband’s name).
6. CNIC Number of the employer.
7. Gender of the employer.
8. Mobile contact number of the employer.
9. E-mail address of the employer.
10. Name, gender, date of birth and relation of employer’s family
members with the employer.
11. Full name of the Manager, if any (including his father’s name).
12. CNIC Number of the Manager.
13. Gender of the Manager.
14. Mobile contact number of the Manager.
15. E-mail address of the Manager.
16. Category of the establishment, i.e., whether a shop, industrial
establishment, commercial establishment, residential hotel, restaurant,
eating house, theatre or other place of public amusement or
entertainment etc.
17. Name of the Chamber of Commerce and Industry with which the
establishment is registered/ affiliated (if any).
18. Total number of employees (state separately the number of men,
women and/or adolescents, if any along with their category as
permanent employee or temporary employee).
19. Date, month and year in which the establishment commenced its work.
20. I hereby declare that the details given above are correct to the best of
my knowledge.
Dated Signature of the employer
Note–This statement shall be sent to the Deputy Chief Inspector of the area
concerned.
(b) in Form B, for the words “Young persons” wherever appearing, the word
“Adolescents” shall be substituted.
(c) for Form C, the following shall be substituted:
85
Substituted by the Punjab Shops and Establishments (Amendment) Act 2023 (VI of 2023), published in the Punjab Gazette
(Extraordinary), dated: 17 February 2023, pp. 3869-3874, s.22.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-331-320.jpg)
![“Registration No.;
Registration Date;
REGISTRATION CERTIFICATE
______________________________________________________________
_______________
It is hereby certified that the establishment---------------------------------------
located at ------------------------------------------TEHSIL---------------------DISTRICT---
------------ has been registered under THE PUNJAB SHOPS AND
ESTABLISHMENT ORDINANCE, 1969.
Registration valid till ------------- Chief Inspector of Shops & Establishments”]](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-332-320.jpg)


























![23 | P a g e
activities, powers and functions and whose compliance shall be binding on the Authority, within
a stipulated time.
(2) Notwithstanding anything contained in sub-section (1) if there is any difficulty in
implementation of the directions and guidelines of the Policy Board or the Federal Government,
the Authority shall refer the case back to the Federal Government for its review specifying
reasons for non-implementation, within the stipulated time, whose decision in this respect shall
be final.
42. Winding up of Authority. No provision of any law relating to winding up of
bodies corporate shall apply to the Authority. The Authority shall only be wound up by the law
to be enacted by the Parliament for winding up of the Authority).
SCHEDULE-I
[See Section 2 (v, xii, xviii, xix, & xxviii)]
1. BIOLOGICALS includes,
(1) Biological drugs produced by biological systems and which require standardization
by biological assays according to the relevant and updated recommendations of the World Health
Organization published in Technical Report Series and Biological Standardization Report and
includes--
(a) blood products including Plasma, Albumin, Clotting Factors, Factors VIII,
IX, Mixed Clotting Factors Tractions, Fibrinogens, Immunoglobulins:
(b) immunological products including Antisera, Antitoxins, specific
Immunoglobulins;
(c) in vivo diagnostics including Tuberculins, Lepronin, Histoplasmin,
Coccidioidin, Allergens, Allergens Extracts, Antibodies conjugated with
isotopes for imaging studies;
(d) antigens, cytokines/antibodies/cells injected to elicit a biological response;
(e) vaccines, including:--](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-359-320.jpg)

![25 | P a g e
stabilization, filling and packaging. The diluted and stabilized bulk requires its own set of quality
control test and the final finished form of such Biological Drugs under go another set of
complete quality control tests. The final product is released by the Pakistan's National Control
Laboratory for Biologicals under the World Health Organization's Lot Release system of
evaluation.
(5) ''Biological Drugs (Naked vials)", are Biologicals Drugs that are defined in sub-
section (1) above that are manufactured and filled at one site but the final containers are neither
labeled nor packed in cartons. These drugs are imported in unlabeled vials and are labeled and
packed in carton locally. In such cases at least an identity test is required to confirm the positive
identification of the required antigen. The final product is released by the Pakistan's National
Control Laboratory for Biologicals under the World Health Organization's Lot Release system of
evaluation.
(6) Originator Biological Drugs means a biological drug which has been licensed by the
national regulatory authorities on the basis of a full registration dossier; i.e. the approved
indication(s) for use were granted on the basis of full quality, efficacy and safety data:
(a) reference biotherapeutic product (RBP) means an originator biological
drug product that was licensed on the basis of a full registration dossier. It
does not refer to measurement standards such as international,
pharmacopoeial, or national standards or reference standards;
(b) biosimilar biological drugs mean Similar Biotherapeutic Product (SBP)
which is similar in terms of quality, safety and efficacy to an already
licensed reference biotherapeutic product;
(c) similarity means absence of a relevant difference in the parameter of
interest.
1
[(7) No human vaccines, blood products (plasma derivatives medicinal products) and
anti-sera (antivenoms and anti-rabies) shall be sold and used until a "Lot Release Certificate"
from the Federal Government Analyst of the National Control Laboratory for Biologicals,
Islamabad has been obtained.]
(8) Pharmaceutical dossier includes a set of documents submitted by a Person for the
registration of a therapeutic good, containing complete information about:
1
Substituted vide Notification No. S.R.O 219(I)/2022 dated 14th
February, 2022](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-361-320.jpg)

![27 | P a g e
2
[(b) abortive and contraceptive substances and agents, disinfectants,
bacteriophages, gelatin capsules and antiseptic solution.]
(c) such substances intended to be used for the destruction or repulsion of
such vermin, insects, rodents and other organism as cause, carry or
transmit disease in human beings or animals or for disinfection in
residential areas or in premises in which food is manufactured,
prepared or kept or stored;
(d) such pesticides as may cause health hazard to the public;
(e) any substance mentioned as monograph or as a preparation in the
Pakistan Pharmacopoeia or the Pakistan National Formulary or the
International Pharmacopoeia or the British Pharmacopoeia or the
British Pharmaceutical Codex or the United States Pharmacopoeia or
the National Formulary of the United States, whether alone or in
combination with any substance exclusively used in the Unani,
Ayurvedic, Homoeopathic, Chinese or Biochemic system of treatment,
and intended to be used for any of the purposes mentioned in sub-
Clauses (a), (b) and (c); and
(f) any other substance which the Federal Government may by notification
in the official Gazette, declare to be a drug for the purpose of this Act.
3. MEDICAL DEVICES include,
(a) instruments, medical equipment, implants, disposables and
software, used mainly for the purpose of diagnosis, monitoring and
treatment of disease, or
(b) any other item which the Federal Government may, by notification
in the official Gazette, declare as medical device;
4. MEDICATED COSMETICS include,
Cosmetics containing drugs and are defined as articles containing active drug
ingredients intended to be rubbed, poured, sprinkled, or- sprayed on, or introduced into, or
2
Substituted vide Notification No. S.R.O 824(I)/2018 dated 26th
June, 2018. Before substitution, sub-paragraph (2) of ,
for sub-para (b) read as under:
“(abortive and contraceptive substances, agents and devices, surgical ligatures, sutures, bandages, absorbent
cotton, disinfectants, bacteriophages, adhesive plasters, gelatin capsules and antiseptic solution;.”](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-363-320.jpg)
![28 | P a g e
otherwise applied to human body or part thereof for cleansing, beautifying, promoting
attractiveness, or altering the appearance, and articles intended for use as a component of any
such articles; except that such term shall not include soap.
SCHEDULE-II
[See Section 2(xxx)]
PROHIBITIONS
A. Import, manufacture and sale of therapeutic goods:
(1) No person shall himself or by any other person on his behalf,—
(a) Export, import or manufacture for sale or sell,—
(i) any spurious therapeutic good;
(ii) any counterfeit therapeutic good;
(iii) any misbranded therapeutic good;
(iv) any adulterated therapeutic good;
(v) any substandard therapeutic good;
(vi) any therapeutic good after its expiry date;
(vii) any therapeutic good which is not registered or is not in
accordance with conditions of registration as disclosed in the
registration dossier and that has undergone pharmaceutical
evaluation;
(viii) any therapeutic good which, by means of any statement, design or
device accompanying it or by any other means, purports or claims
to cure or mitigate any such disease or ailment, or to have any such
other effect, as may be prescribed;
(ix) any drug if it is dangerous to health when used in the dosage or
with the frequency, or for the duration specified, recommended or
suggested in the labeling thereof; or
(x) any therapeutic good in contravention of any of the provisions of
this Act or rules made thereunder;](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-364-320.jpg)

![30 | P a g e
(ii) in the case of an imported drug, by the manufacture or
importer of that therapeutic good or if the therapeutic good
is imported through an indenter by such indenter; and
(iii) apply an incorrect batch number to a therapeutic good.
(2) Nothing in Paragraph (1) shall apply to the manufacture of small quantities
of any therapeutic good for the purpose of clinical trial examination, test, analysis or
personal use in small quantities.
3
[(3) No person shall himself or by any other person on his behalf sell or offer for
sale, any drug in the finished form over and above the maximum retail price as may be
fixed by the Federal Government or determined under the provisions of the Drug Pricing
Policy, as notified by the Authority.
Explanation.- For the purpose of this paragraphs, the drug shall also include
biologicals.]
B. Control of advertisement:—
No person shall himself or by any other person on his behalf advertise,
except in accordance with such conditions as may be prescribed,—
(a) any therapeutic good;
(b) any substance used or prepared for use in accordance with the
Ayurvedic, Unani, Homoeopathic, Chinese or Biochemic system
of treatment or any other substance or mixture of substances as
may he prescribed;
(c) any remedy, treatment or offer of a treatment for any disease.
Explanation.--For the purpose of this entry "Advertise" means to
make any representation by any means whatsoever for the purpose
of promoting directly or indirectly the sale or disposal of
therapeutic good, a substance or a mixture of substances, a
remedy or a treatment except the display of sign boards for a
clinic, a dispensary or a hospital or such other institution offering
treatment.
3
Added vide Notification No. S.R.O 913(I)/2017 dated 6th
September, 2017.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-366-320.jpg)
![31 | P a g e
C. Control of samplings:—
No person shall distribute or cause to be distributed any therapeutic good as a
sample except in accordance with such conditions as may be prescribed.
D. Control of printing of labeling:—
No person shall print any label in respect of any therapeutic good which is
required to be registered under this Act but is not so registered after the date fixed by the
Federal Government under sub-section (6) of Section 7 of Act, or for a person who does
not possess a license under that Act to manufacture that therapeutic good.
SCHEDULE-III
[See Section 27]
OFFENCE
(1) Whoever himself or by any other person on his behalf,—
(a) exports, imports, manufactures for sale or sells any spurious
therapeutic good or any therapeutic good which is not registered;
(b) manufactures for sale any therapeutic good without a license;
(c) imports without license any therapeutic good for the import of
which a license is required;
Shall be punishable with imprisonment for a term which
shall not be less than three years or more than ten years and with
fine which may extend to ten lakh rupees:
Provided that the Drug Court may, for any special reasons
to be recorded, award a sentence of imprisonment for a term of
less than three years.
(2) Whoever himself or by any other person on his behalf,--](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-367-320.jpg)

![33 | P a g e
section shall be punishable with imprisonment for life or with imprisonment which shall
not be less than five years and with fine which may extend to five hundred thousand
rupees.
(6) Penalty for violating the prohibitions.--Whoever himself or by any other
person on his behalf violates any prohibitions specified in Schedule-II shall be punished
with imprisonment for a term up to five years and with fine up to five hundred thousand
rupees.
(7) Recovery for overcharging.- Where any person has been convicted under the
provisions of paragraph (3) of heading “A” of Schedule-II, he shall be liable to the
punishment as below.-
(i) a fine equal to overcharged amount based on country-wide average sale of
the respective drug in the last three financial years along-with 20%
surcharge, if he is a manufacturer or importer;
(ii) a fine of rupees 02 million to 10 million, if he is a distributor;
(iii) a fine of rupees 0.1 million to 01 million, if he is a retailer.
Provided that all amounts due from a person under this paragraph shall be
deposited in the Government treasury.
Explanation.- For the purpose of this paragraph, the drug shall also include
biologicals.4
SCHEDULE-IV
[See Section 29]
COGNIZANCE OF OFFENCES
(1) Subject to the provisions of Schedule-V no prosecution shall be instituted
under this Act except,—
(a) by a Federal Inspector, where the prosecution is in respect
of a contravention of Clause (h) of Paragraph (1) of heading A of
Schedule-II or any of the provisions of this Act or the rules
4
Added vide Notification No. S.R.O 913(I)/2017 dated 6th
September, 2017.](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-369-320.jpg)
![34 | P a g e
relating to the import or export of therapeutic goods or the
manufacture for sale, or sale, of a therapeutic good which is not
for the time being registered or for the manufacture for sale of
which a license is not for the time being in force; or
(b) by a Provincial Inspector:
Provided that, where the public interest so requires, the
Federal Inspector may, with the prior permission of the
Registration Board or Licensing Board as the case may be,
institute a prosecution for a contravention of any other provision
of this Act and The Drugs Act, 1976 (XXXI of 1976).
(2) Notwithstanding anything contained in the Code of Criminal Procedure, 1898
(Act V of 1898):
(a) an offence punishable under Schedule-III other than an offence
mentioned in Clause (1) of that Schedule shall be non-cognizable,
and
(b) no Court other than a Drug Court established under The Drugs
Act, 1976, (XXXI of 1976) shall try an offence punishable under
this Act and Drugs Act, 1976 (XXXI of 1976);
(c) nothing contained in this Schedule shall be deemed to prevent any
person from being prosecuted under any other law for any act or
omission which constitutes an offence punishable under this Act
or The Drugs Act, 1976 (XXXI of 1976) or to require the
transfer to a drug Court of any case which may be pending in
any Court immediately before the establishment of Drug Court.
SCHEDULE-V
[See Section 2(xvi)]
POWERS OF INSPECTORS
(1) Subject to the provisions of this Schedule and of any rules made in this
behalf, an Inspector may, within the local limits for which he is appointed, and in any](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-370-320.jpg)



![38 | P a g e
Board or the Registration Board, as the case may be, before which proceedings are
instituted or action is initiated in respect of the drug.
(5) Where an Inspector takes any action under section this Schedule,--
(a) he shall as soon as practicable ascertain whether or not the
therapeutic good contravenes any of the provisions of this Act
and, it is ascertained that the drug does not so contravene, he
shall forthwith revoke the order passed under the said section or,
as the case may be, take such action as may be necessary for the
return of the stock seized and payment for the samples taken,
under intimation to the Board concerned;
(b) if he seizes the stock of the therapeutic good he shall, as soon as
may be inform the Board concerned and take its order as to the
custody thereof:
Provided that where a Federal Inspector is not competent to take
action under Schedule-IV, he shall as soon as may be, report the matter
and hand over the stock, if any, to the Provincial Inspector for further
action under this Act or The Drugs Act, 1976.
(6) The Provincial Inspector on finding any contravention of this Act or the
Drugs Act, 1976 (XXXI of 1976) shall, unless the Board otherwise directs, always refer
the case to the Provincial Quality Control Board and seek orders as to the action to be
taken in respect of such contraventions.
(7) The Federal Inspector on finding any contravention of this Act or the Drugs
Act, 1976 (XXXI of 1976) for which he is authorized shall unless otherwise directed,
always refer the case to the Licensing Board or the Registration Board or any other
authority as may be specified for the purpose and seek any further orders as to the action
to be taken in respect of such contravention.
SCHEDULE-VI
[See Section 2(i)]
(1) The Drugs Act, 1976 (XXXI of 1976).
(2) Rules made under the Drugs Act, 1976 (XXXI of 1976).
--------------------------](https://image.slidesharecdn.com/forensicpharmacyfinalprofessionalcompletesyllabus-240929082329-5452b58c/85/Forensic-Pharmacy-Final-Professional-Complete-Syllabus-PharmD-pdf-374-320.jpg)