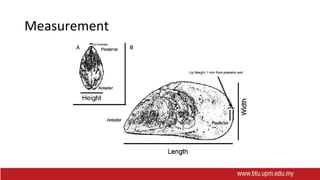
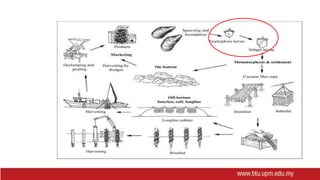
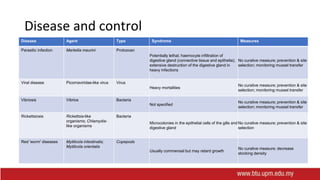
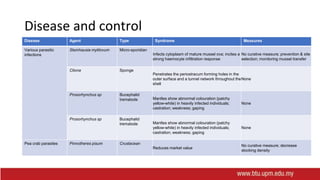
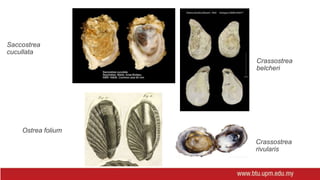
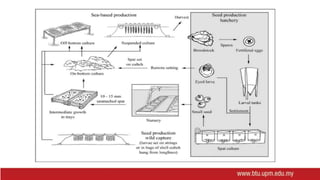
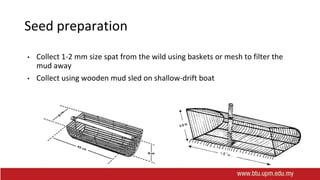
This document provides information on mollusc culture, including mussels, oysters, and cockles. It discusses the biological features and life cycles of these molluscs and outlines best practices for their cultivation, including site selection, seed collection and preparation, nursery and grow-out methods, harvesting, and issues to consider. The key steps involve natural seed collection or hatchery production, nursery culture, transfer to intertidal or subtidal grow-out using various suspension or bottom methods, and harvesting at market size, typically after 18-24 months. Disease prevention and control are also important considerations.