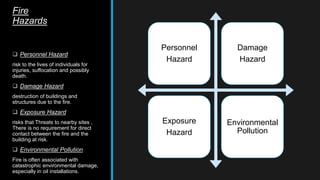
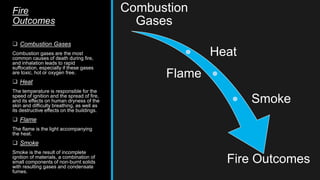
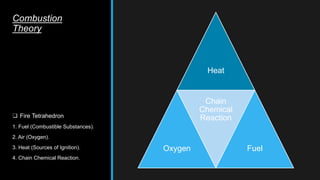
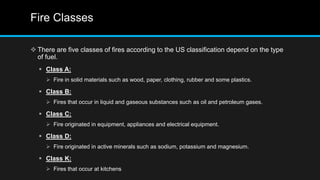
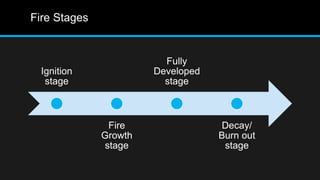
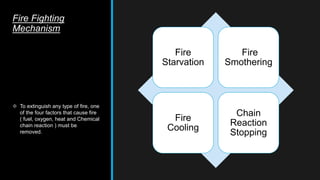
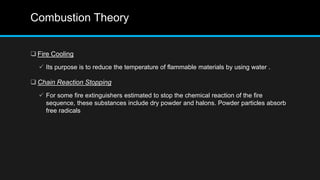
This document provides an overview of fire, including its definition, causes, outcomes, classes, stages, combustion theory, and firefighting mechanisms. It defines fire as a rapid oxidation chemical reaction and notes that fires usually start small due to sparks from neglecting prevention. The main outcomes of fire are combustion gases, heat, flames, and smoke. Fires can be caused by human carelessness, natural causes, or technical failures. Combustion requires fuel, oxygen, heat, and a chain reaction. Firefighting works by removing one of these factors through starvation, smothering, cooling, or stopping the chain reaction.