1. Cervical cancer is the fourth most common cancer in women worldwide. It arises from the transformation zone of the cervix where the squamous and glandular epithelia meet.
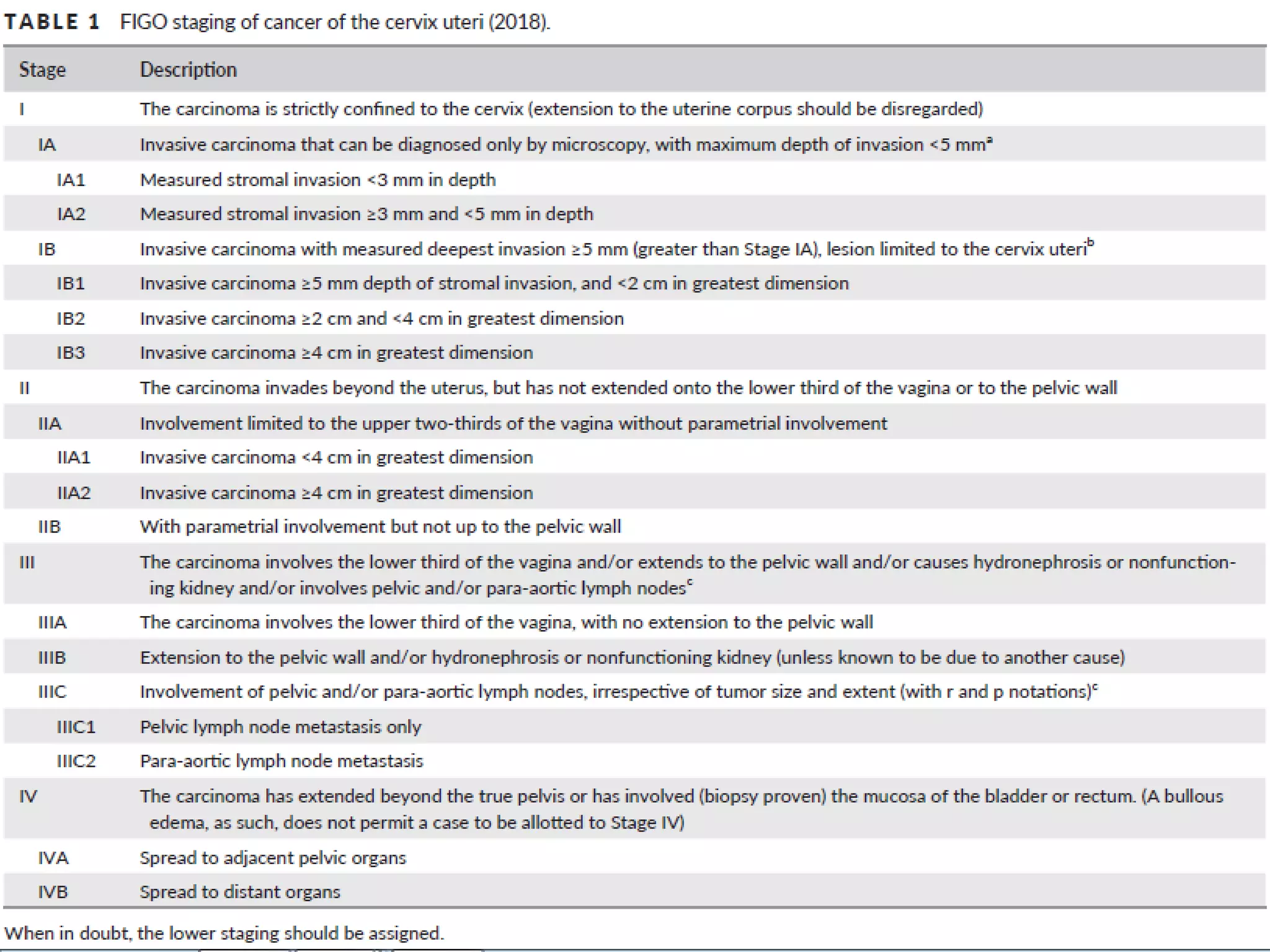
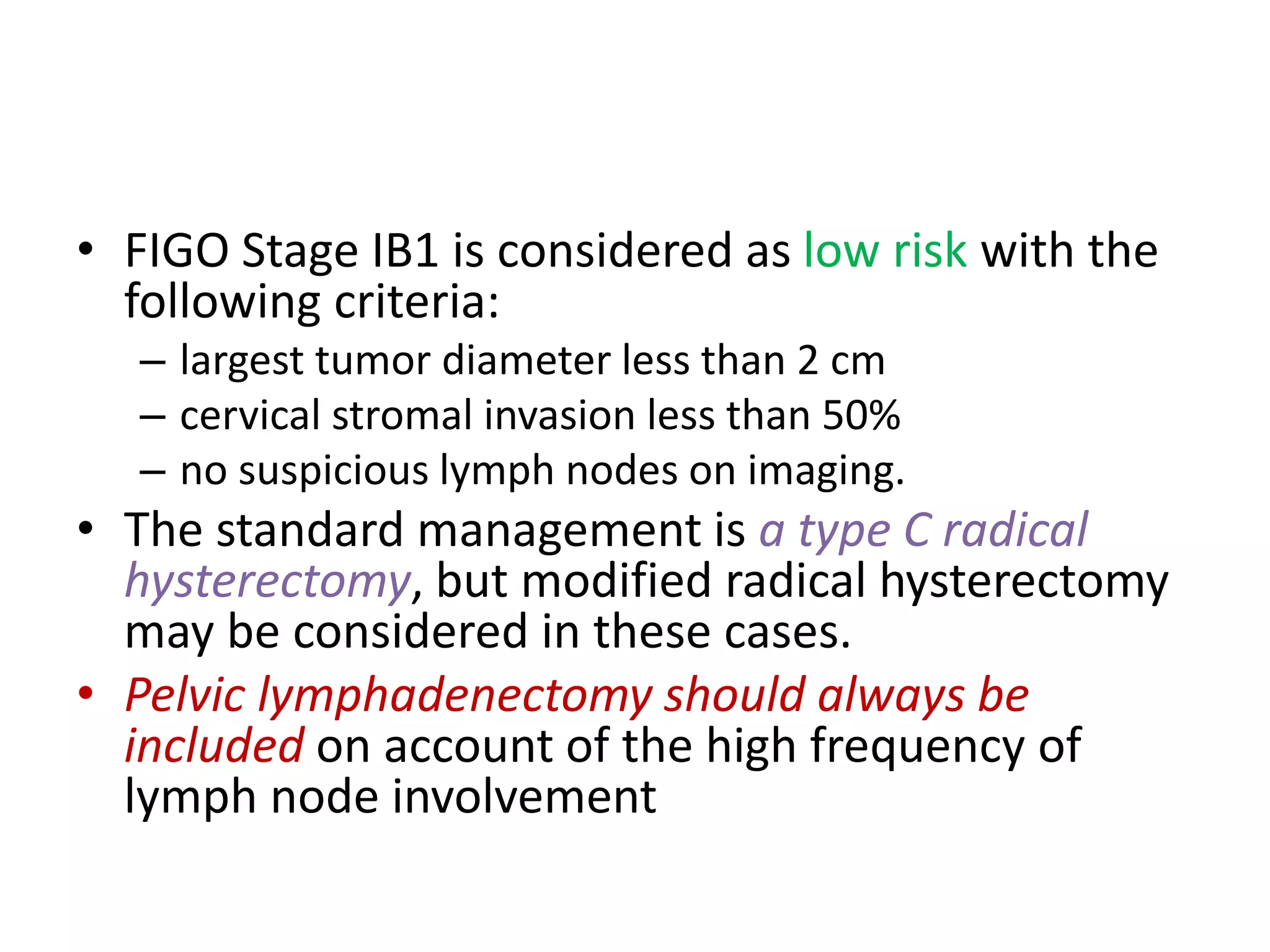
2. FIGO stage IB1 cervical cancer with tumors less than 2 cm are considered low risk if stromal invasion is less than 50% and lymph nodes are negative. The standard treatment is a type C radical hysterectomy with pelvic lymphadenectomy.
3. For young women wishing to preserve fertility, a radical trachelectomy may be performed for stage IA2-IB1 tumors less than 2 cm, which removes the cervix while maintaining the uterus. Pelvic lymph node assessment is also required.