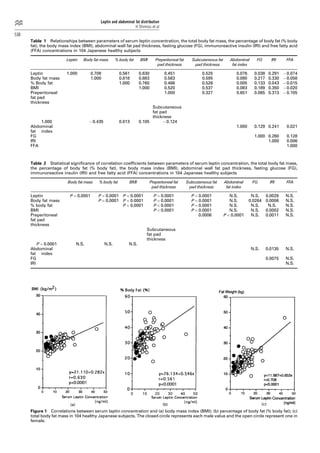
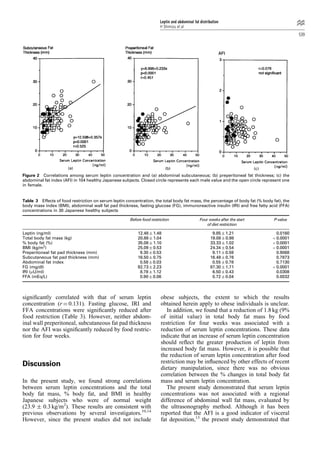
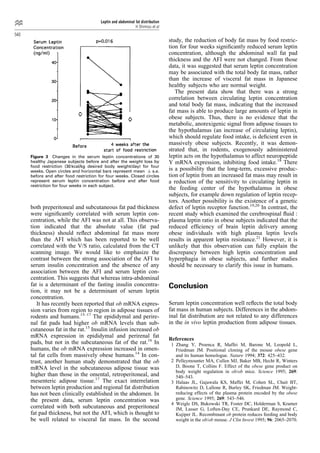
This study investigates the relationship between serum leptin concentrations and body fat parameters in 104 healthy Japanese subjects. Results show that serum leptin is significantly correlated with total body fat mass, body fat percentage, and BMI, but not with abdominal fat distribution. A four-week food restriction reduced both serum leptin levels and body fat mass, although the percentage change in leptin did not correlate with the change in body fat.