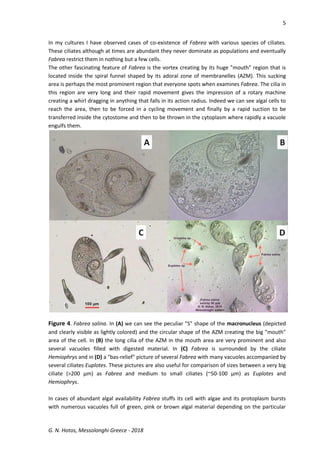
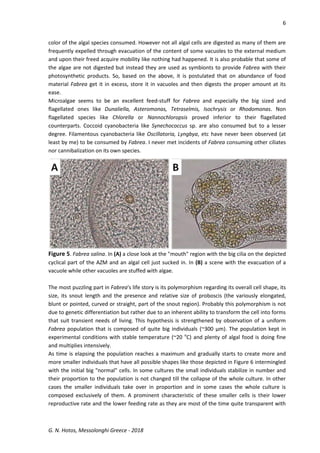
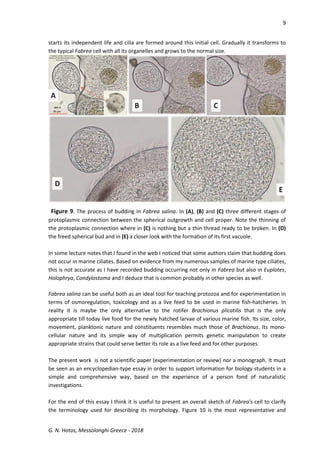
This document discusses the ciliate protozoan Fabrea salina, prevalent in hypersaline waters, detailing its culturing challenges, morphological characteristics, and behaviors. It highlights the organism's ability to thrive in various salinities, its feeding habits, and the notable polymorphism of its cell forms. The author also observes interactions with other ciliates and provides insights into laboratory culturing techniques and the organism's ecological significance.