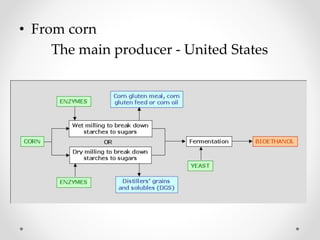
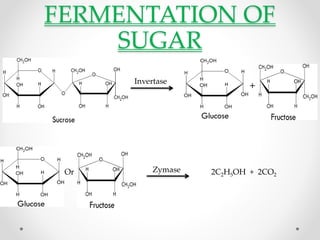
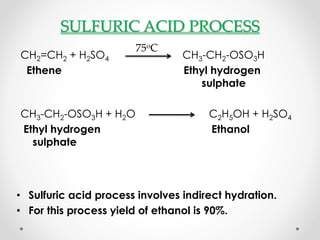
This document summarizes information about ethanol, including its molecular formula, physical properties, methods of production, and flammability. Ethanol can be produced from biomass like sugar, starch, and cellulose via acid-catalyzed processes or from acetylene. The primary production methods are the sulfuric acid process and phosphoric acid process. Ethanol is also commonly produced via fermentation of sugar or biomass like corn, sugar cane, and wheat. The document provides details on ethanol production from various feedstocks and outlines some major global producers.