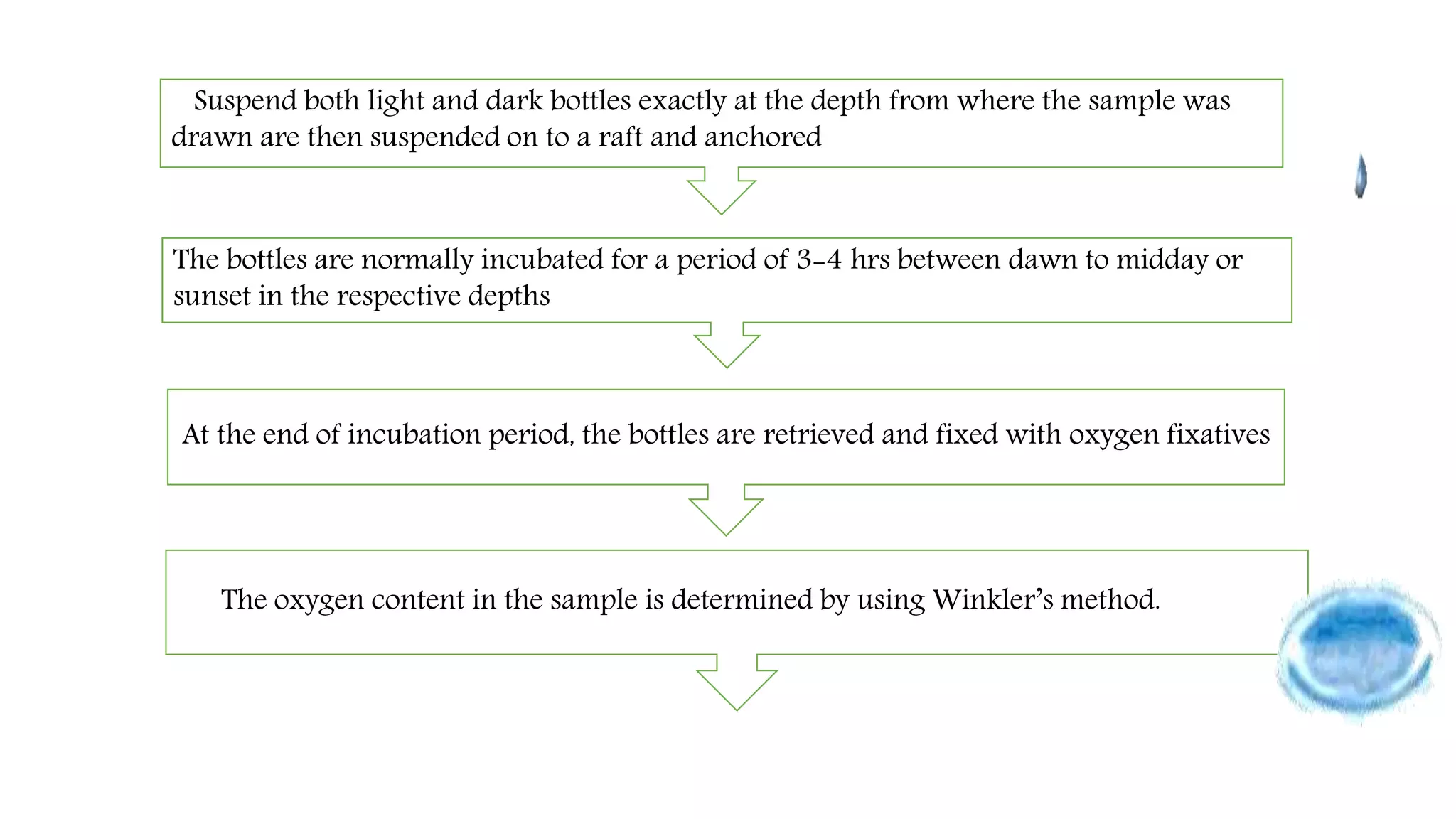
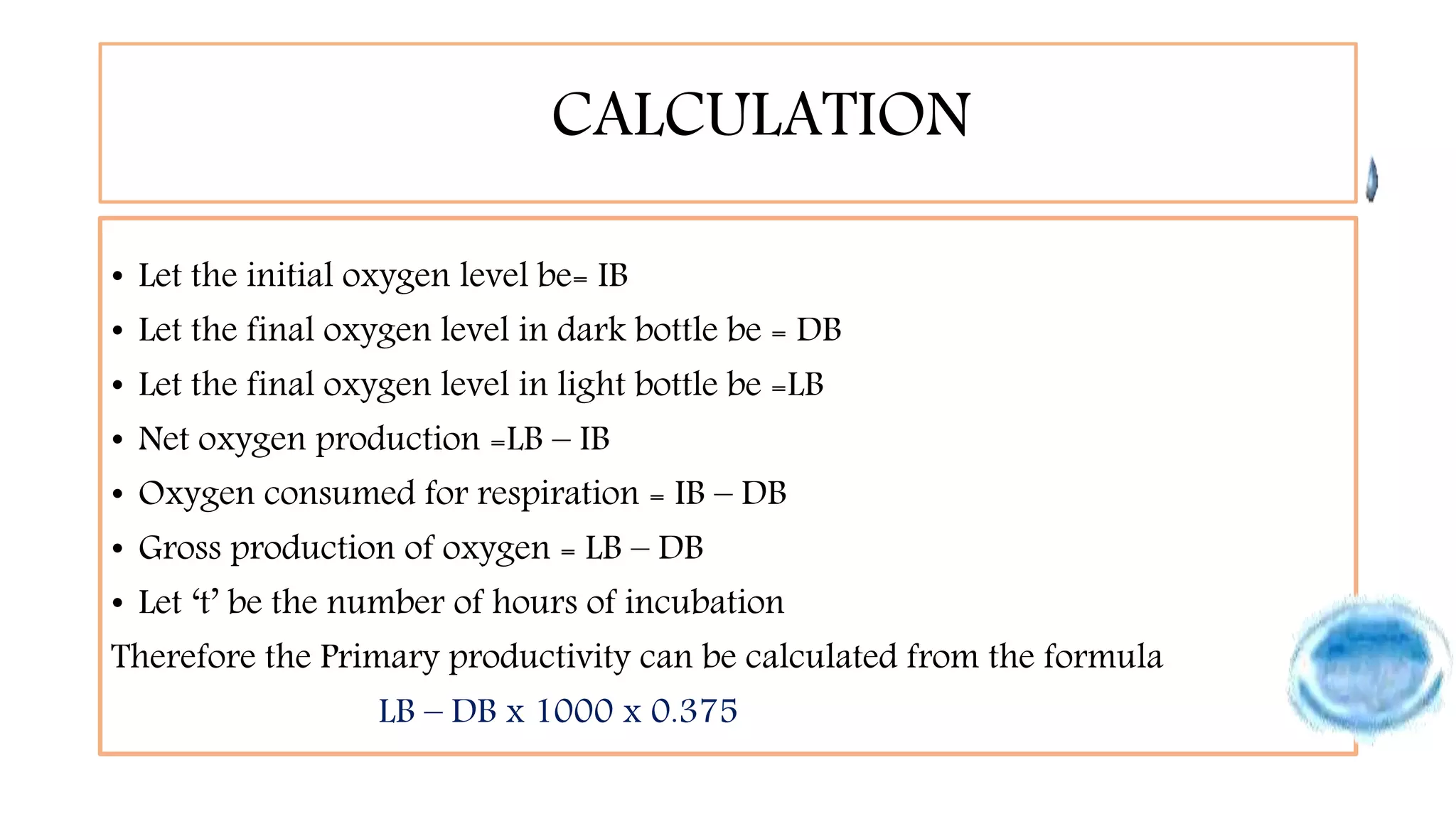
This document describes the procedure for estimating primary productivity of freshwater bodies. Water samples are collected in light and dark bottles, with one bottle serving as a control. The bottles are incubated for 3-4 hours, then the oxygen content is measured using Winkler's method. Primary productivity is calculated based on the difference between oxygen levels in the light and dark bottles, accounting for respiration. Gross primary productivity is estimated as the change in oxygen from dark to light bottles, while net primary productivity excludes respiration.