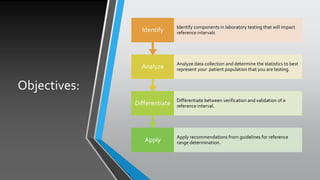
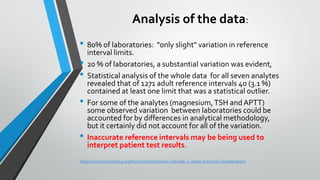
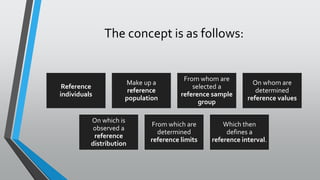
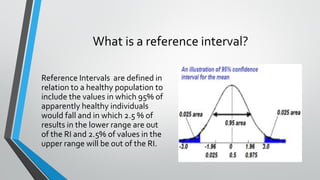
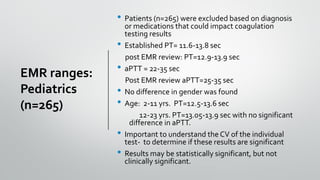
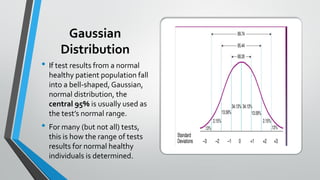
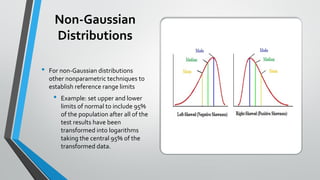
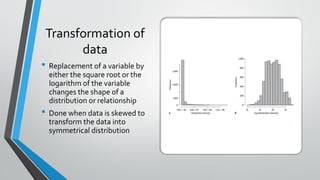
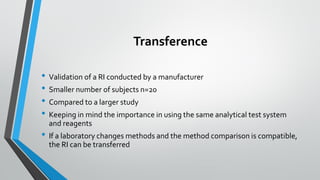
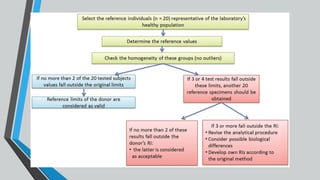
The document outlines the importance of establishing accurate reference intervals for laboratory tests, emphasizing the need for laboratories to conduct their own studies rather than relying on manufacturer data. It also discusses current practices and challenges in reference interval determination, highlighting the variations in methods used and the lack of internal validation in many laboratories. Key considerations include the need for representative patient populations, the impact of biological variation, and the necessity of adapting reference intervals for specific demographics, including pediatrics and geriatrics.