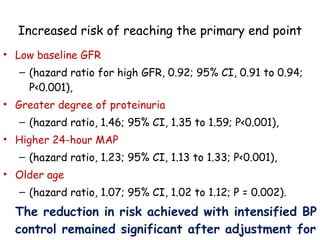
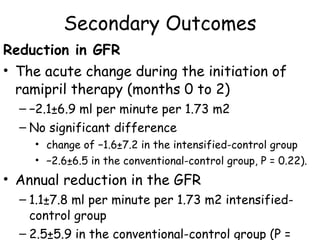
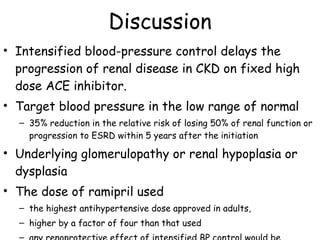
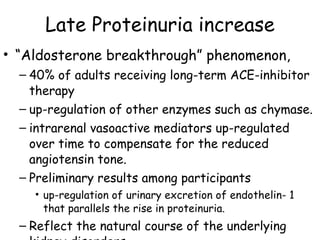
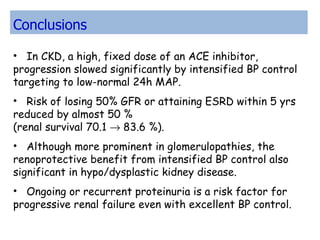
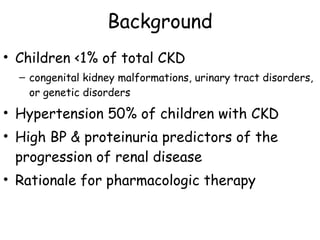
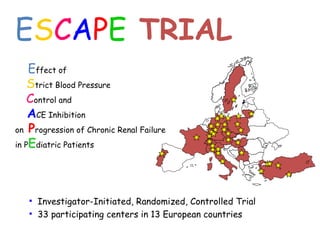
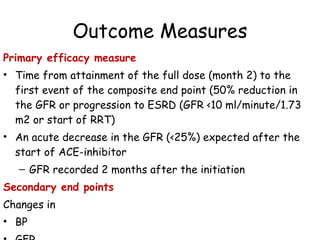
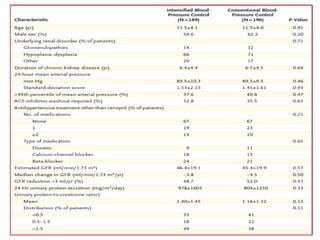
The ESCAPE trial was a randomized controlled trial investigating whether intensified blood pressure control aimed at achieving low-normal 24-hour blood pressure levels could slow the progression of chronic kidney disease in children receiving ACE inhibitor therapy. The trial found that intensified blood pressure control reduced the risk of kidney function decline or end-stage renal disease by 35% over 5 years compared to conventional blood pressure control. Additional benefits included reduced proteinuria levels initially and lower blood pressure overall. However, proteinuria increased again over time, suggesting potential aldosterone breakthrough requiring additional treatment. The results provide evidence that tight blood pressure control can protect kidney function in children with chronic kidney disease.





















![Primary End Point 46/182 patients in intensified control group 69/190 in conventional-control group, progressed to the primary end point, corresponding to an 5-year rate of delay in the progression of renal disease of 70.1% vs 58.3% (P = 0.02 log-rank test) Hazard ratio for progression to the end point with intensified control 0.65 (95% CI], 0.44- 0.94; P = 0.02)](https://image.slidesharecdn.com/escape-110222230008-phpapp02/85/Escape-26-320.jpg)