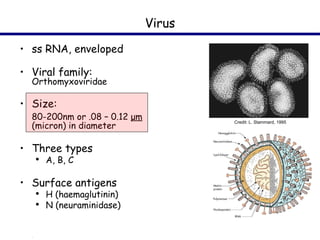
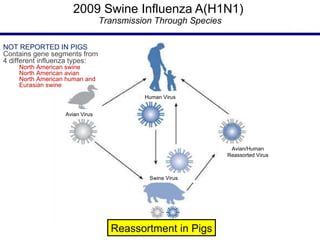
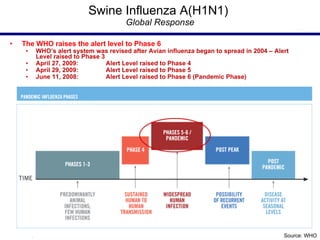
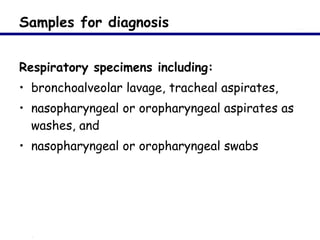
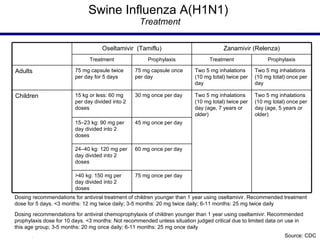
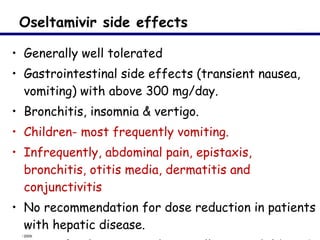
This document provides an outline on influenza virus including definitions, types, transmission, treatment guidelines, and the 2009 H1N1 pandemic. It discusses the virus structure and types A, B, and C. It outlines seasonal flu, pandemics, and the 2009 H1N1 outbreak in Mexico and the US. Treatment guidelines recommend antiviral medications like oseltamivir and zanamivir. It also discusses case definitions, personal protective equipment, and infection control measures.






















![Anti Viral medications Resistant to Amanatidine and Rimanatidine Neuraminidase inhibitors available Oseltamivir (Tamiflu) [ Rs 2250 for ten tablets] Zanamivir (Relenza)](https://image.slidesharecdn.com/seminar-091221124711-phpapp01/85/H1N1-33-320.jpg)












![Identified isolation facilities DELHI Yellow Fever Quarantine Centre, Near AAI Residential Colony, New Delhi [APHO- 25652129, Dr S.K Singh:09868252314] Influenza Ward, Ward no 5, Second Floor, New Building, RML Hospital, Delhi-1](https://image.slidesharecdn.com/seminar-091221124711-phpapp01/85/H1N1-51-320.jpg)
