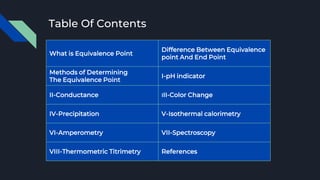
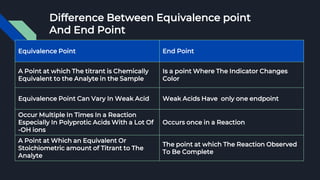
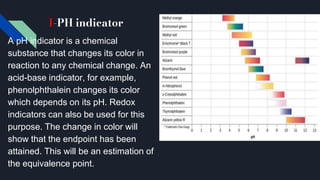
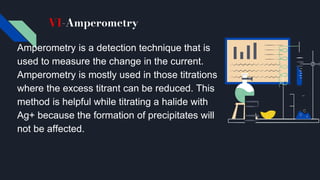
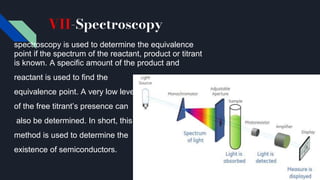
The document explains the equivalence point in titration, where equal quantities of reactants chemically react, particularly in acid-base neutralization. It distinguishes between the equivalence point and the endpoint, the latter being where indicators change color. Various methods for determining the equivalence point are outlined, including pH indicators, conductance, color change, precipitation, isothermal calorimetry, amperometry, spectroscopy, and thermometric titrimetry.