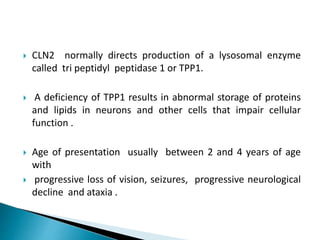
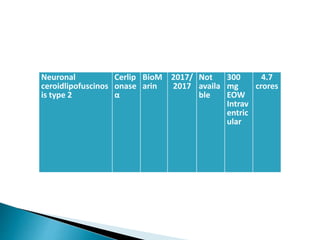
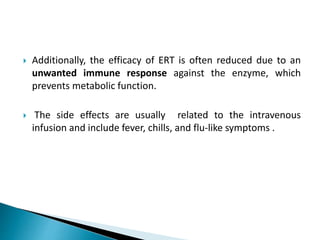
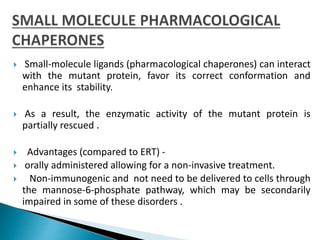
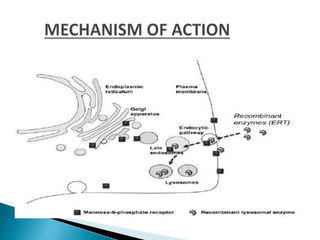
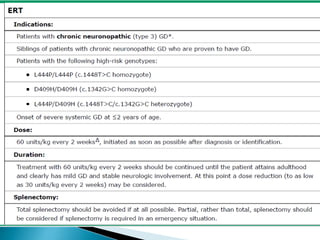
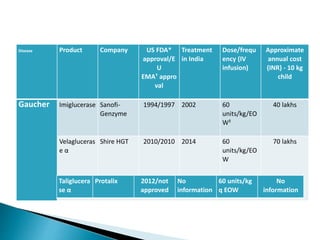
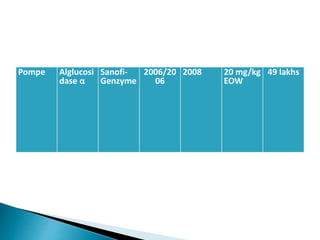
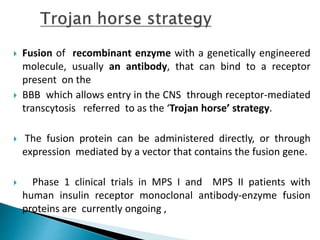
This document discusses enzyme replacement therapy (ERT) for lysosomal storage disorders. It provides details on ERT including its development, mechanisms, available products, dosing and costs. Challenges with ERT include limited blood-brain barrier penetration and immunogenicity. Alternative therapies discussed include substrate reduction therapy, pharmacological chaperones, and direct delivery of enzymes into the cerebrospinal fluid. ERT remains the standard treatment but has limitations for treating neurological manifestations of lysosomal storage disorders.



















![ A possible way to circumvent the BBB is direct delivery of
the enzyme in the cerebrospinal fluid (CSF) through
either intra cerebro ventricular (ICV) injection into the lateral
ventricle (via a catheter/reservoir)
or intrathecal (IT) injection into the lumbar spine or
subarachnoid space at the cisterna magna .
(via lumbar puncture or an IT drug delivery device [IDDD]).](https://image.slidesharecdn.com/enzymereplacementtherapyinneurologicaldisdorders-200607172715/85/Enzyme-replacement-therapy-in-neurological-disorders-31-320.jpg)

![ A rare genetic disorder, belongs to a group of progressive
degenerative neuro metabolic disorders known as the neuronal
ceroid lipofuscinoses (NCL) .
That is characterized by abnormal accumulation of certain fatty,
granular substances (i.e., pigmented lipids [lipo pigments] ceroid
and lipofuscin) within neurons of the brain as well as
other tissues of the body that may result in progressive
deterioration (atrophy) of certain areas of the brain and
neurological impairment.](https://image.slidesharecdn.com/enzymereplacementtherapyinneurologicaldisdorders-200607172715/85/Enzyme-replacement-therapy-in-neurological-disorders-33-320.jpg)