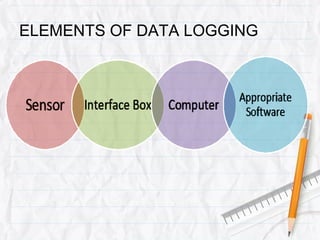
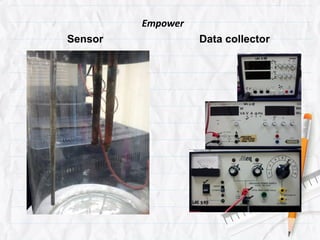
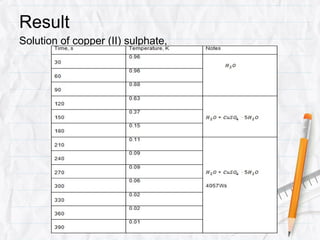
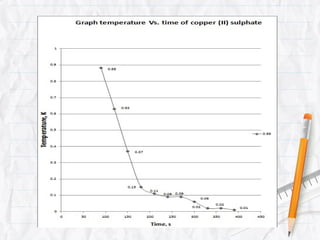
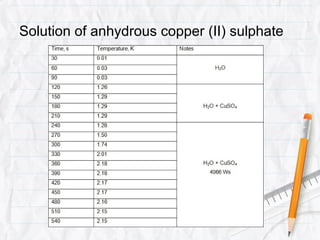
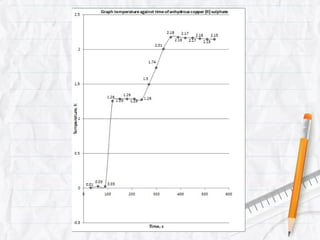
Data logging is the process of using computers to collect data through sensors, analyze the data, and save the results of the collection and analysis. It involves sensors that detect and record measurements, a data collector that receives information from the sensors, and data analysis to interpret the results. Common applications of data logging include monitoring industrial processes, scientific experiments, machine testing, and more.