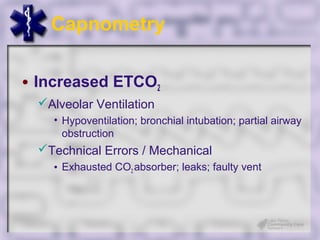
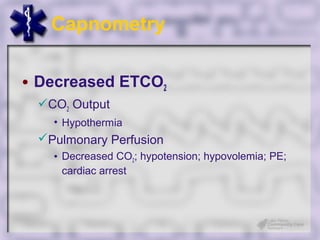
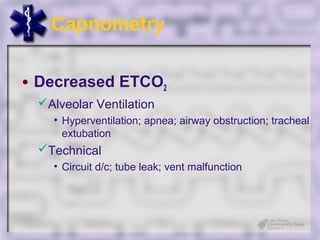
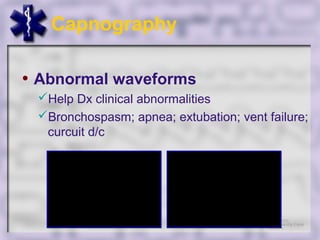
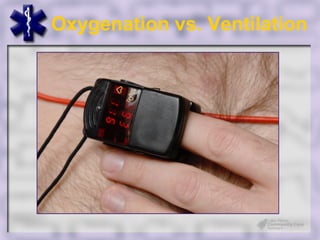
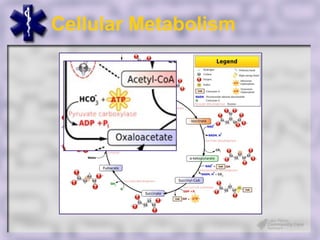
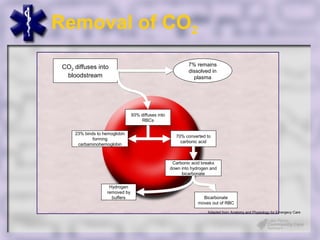
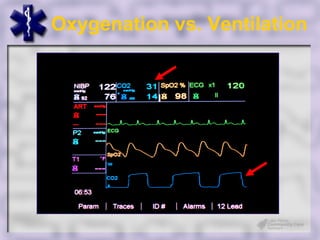
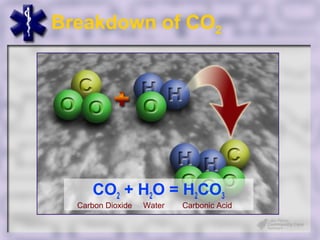
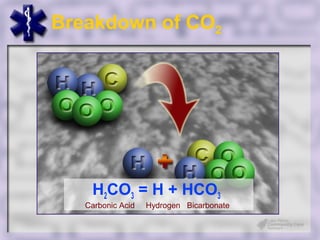
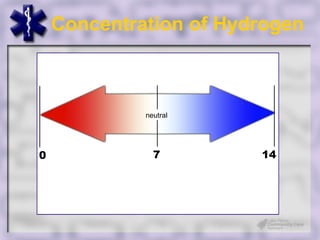
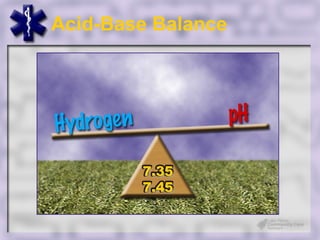
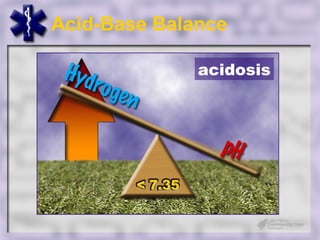
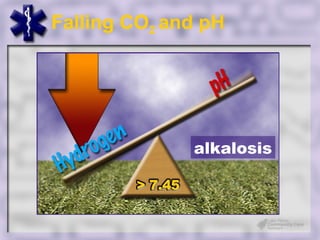
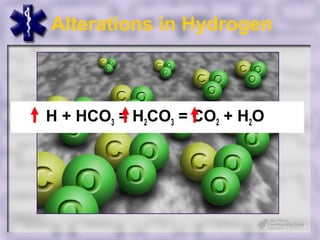
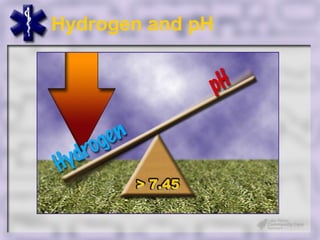
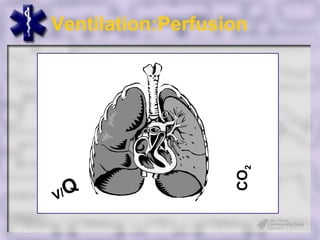
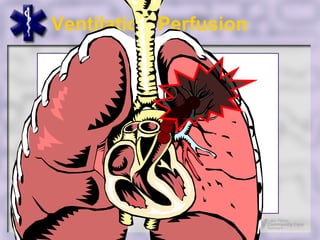
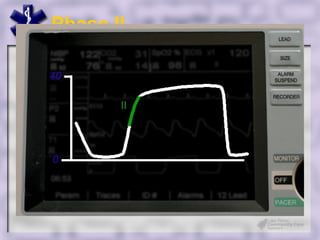
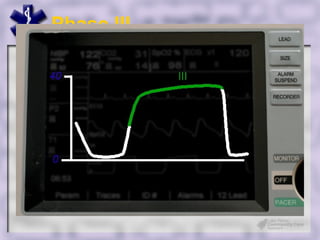
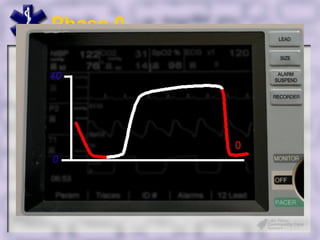
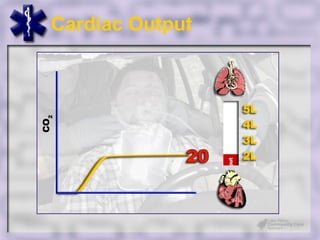
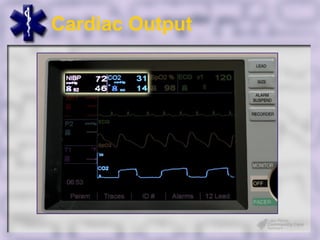
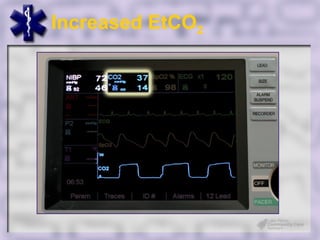
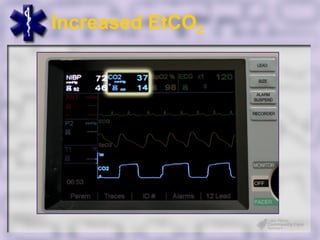
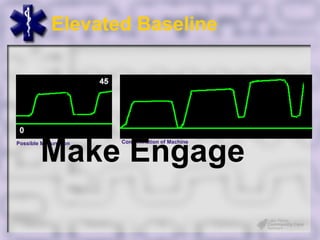
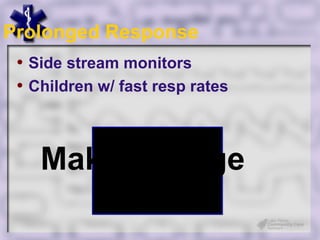
This document provides an overview of capnography and carbon dioxide physiology. It discusses how capnography can be used to ensure proper endotracheal tube placement and monitor ventilation during CPR or respiratory issues. The document outlines carbon dioxide transport in the body and how the capnography waveform can be analyzed to detect abnormalities. In summary, capnography is a vital tool for airway management and ventilation monitoring that provides important real-time patient information.