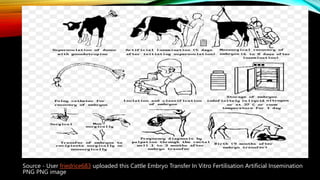
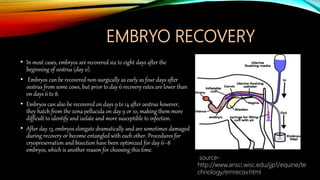
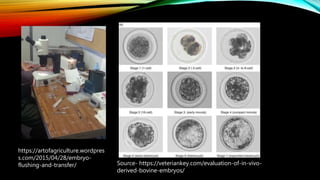
Embryo transfer in cattle involves removing embryos from donor cows and transferring them to recipient cows in order to propagate desirable genetic traits. Key steps include superovulating donors to produce multiple embryos, artificially inseminating donors, flushing embryos from donors 6-8 days later, evaluating and cryopreserving embryos, synchronizing recipients, and surgically or non-surgically transferring embryos into recipients. Embryo transfer allows for genetic improvement of cattle herds, planned mating of elite animals, and circumvention of infertility issues.