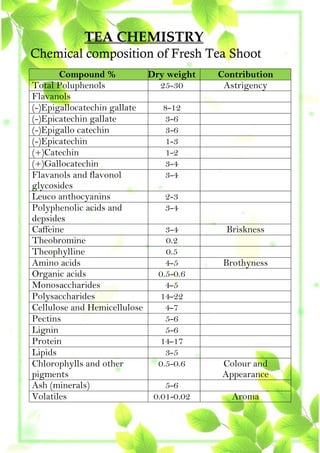
This project by Rema Deosi Sundi from Oxford Public School examines the chemical composition of various tea brands and their flavor profiles, focusing on components like caffeine, tannic acid, and polyphenols. Experiments demonstrate that higher concentrations of polyphenols, tannic acid, and caffeine correlate with better flavor in tea. The findings emphasize the complexity of tea's chemical makeup and its effects on flavor and health benefits.