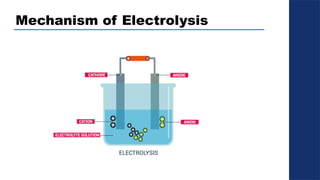
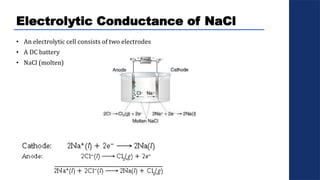
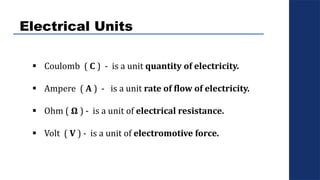
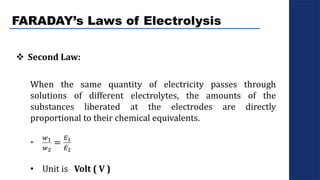
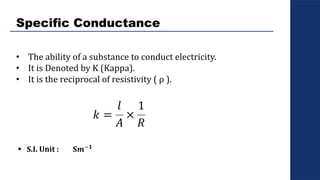
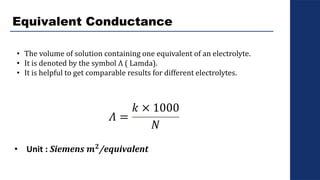
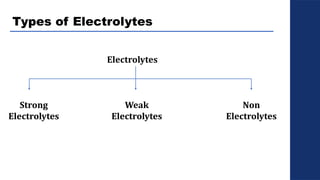
The document discusses electrolysis, detailing its processes, applications, and fundamental principles such as Faraday's laws and electrical units. It explains the conductivity of different electrolytes, emphasizing the differences between strong electrolytes, weak electrolytes, and non-electrolytes. The authors explore factors influencing conductivity and methods for measuring electrolysis efficiency, providing a comprehensive overview of electrotechnical principles in solution chemistry.