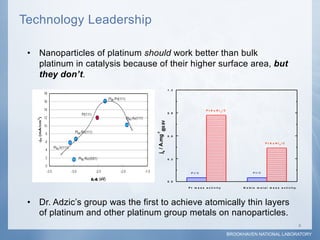
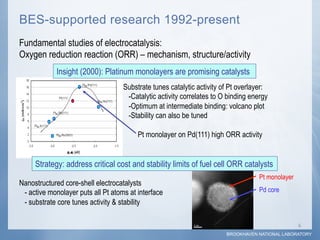
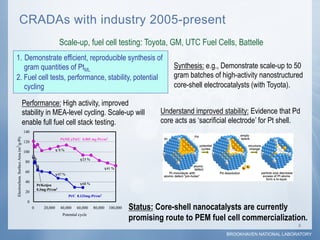
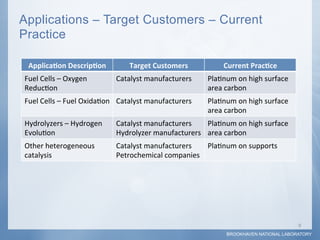
1) Researchers at Brookhaven National Laboratory developed a new type of electrocatalyst using core-shell nanoparticles with a palladium or palladium alloy core coated with a single layer of platinum atoms.
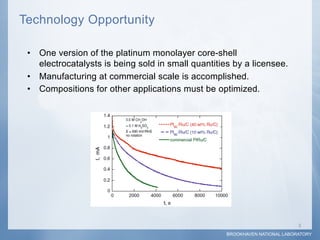
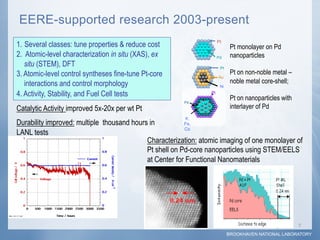
2) Testing showed the platinum monolayer core-shell electrocatalysts improved catalytic activity for fuel cells by 5-20 times compared to bulk platinum per weight of platinum used.
3) Collaboration between the Department of Energy, Brookhaven National Laboratory, and industry partners helped optimize synthesis techniques, test fuel cell performance, and demonstrate the potential for scale-up of production.