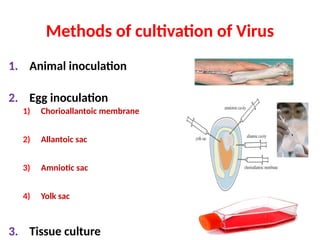
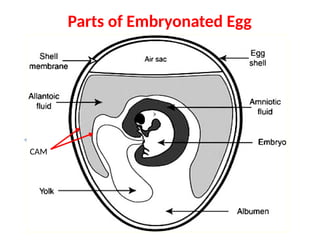
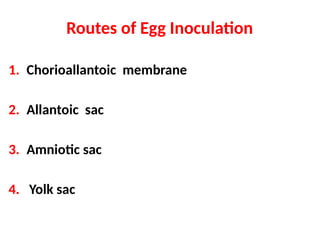
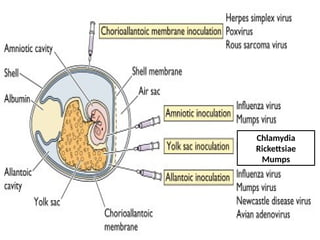
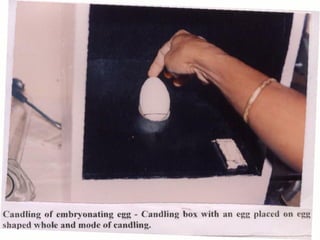
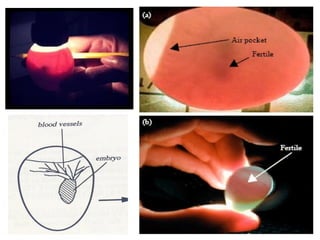
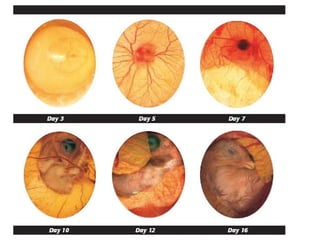
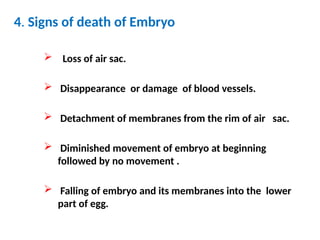
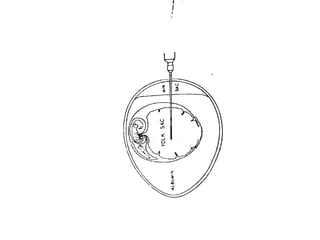
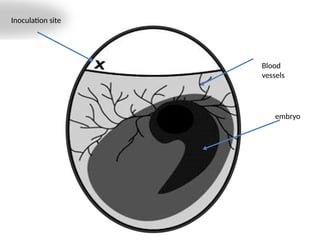
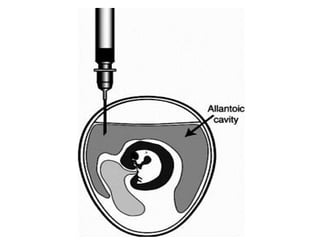
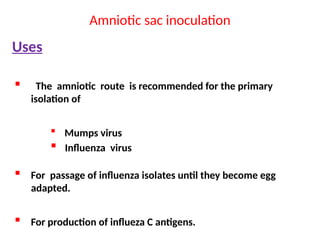
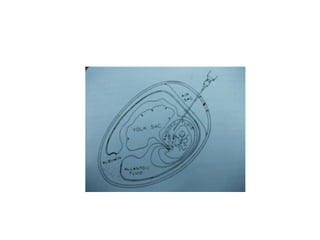
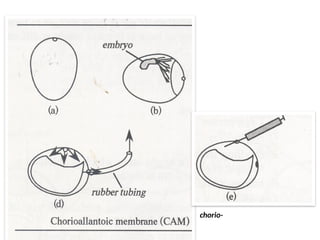
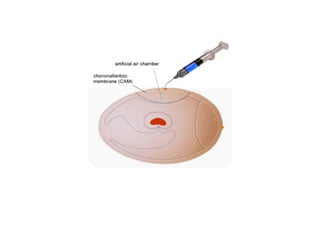
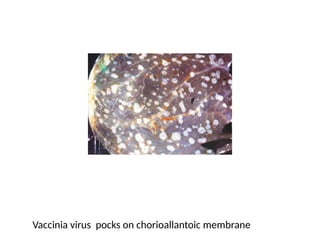
The document outlines the principles and techniques of egg inoculation for virus cultivation, highlighting methods such as chorioallantoic membrane, allantoic sac, amniotic sac, and yolk sac inoculation. It details the advantages of using eggs for virus propagation, including their ideal sterile environment and ease of observation during embryo development. Additionally, it provides instructions for inoculation, incubation parameters, harvesting methods, and monitoring signs of viral growth.