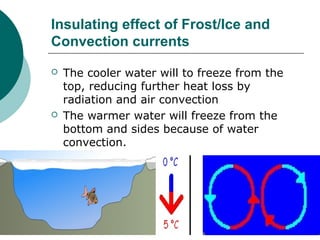
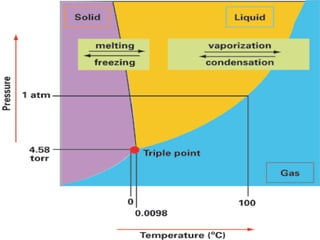
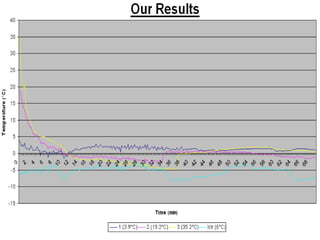
The document summarizes the Mpemba effect, where warmer water sometimes freezes faster than colder water. It describes the effect's history, proposed explanations, and variables that affect outcomes. These include freezing method, definition of "frozen," container properties, and temperature measurement. The authors conducted an experiment demonstrating the effect and found the initially warmer samples froze first. While insulation from early frost formation was initially proposed as the cause, videos suggest it may not fully explain the phenomenon. The causes remain unclear and an area of ongoing research.