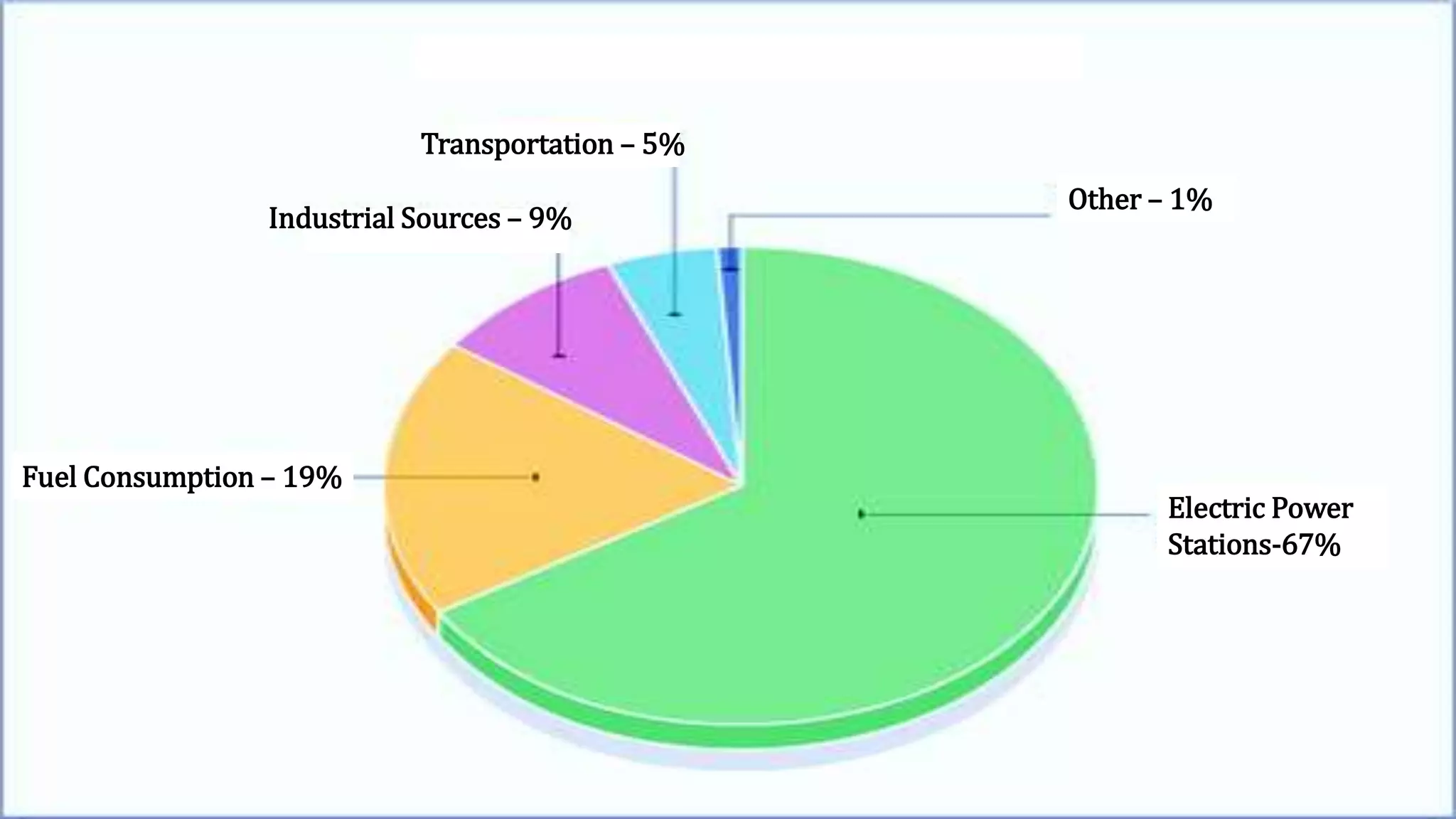
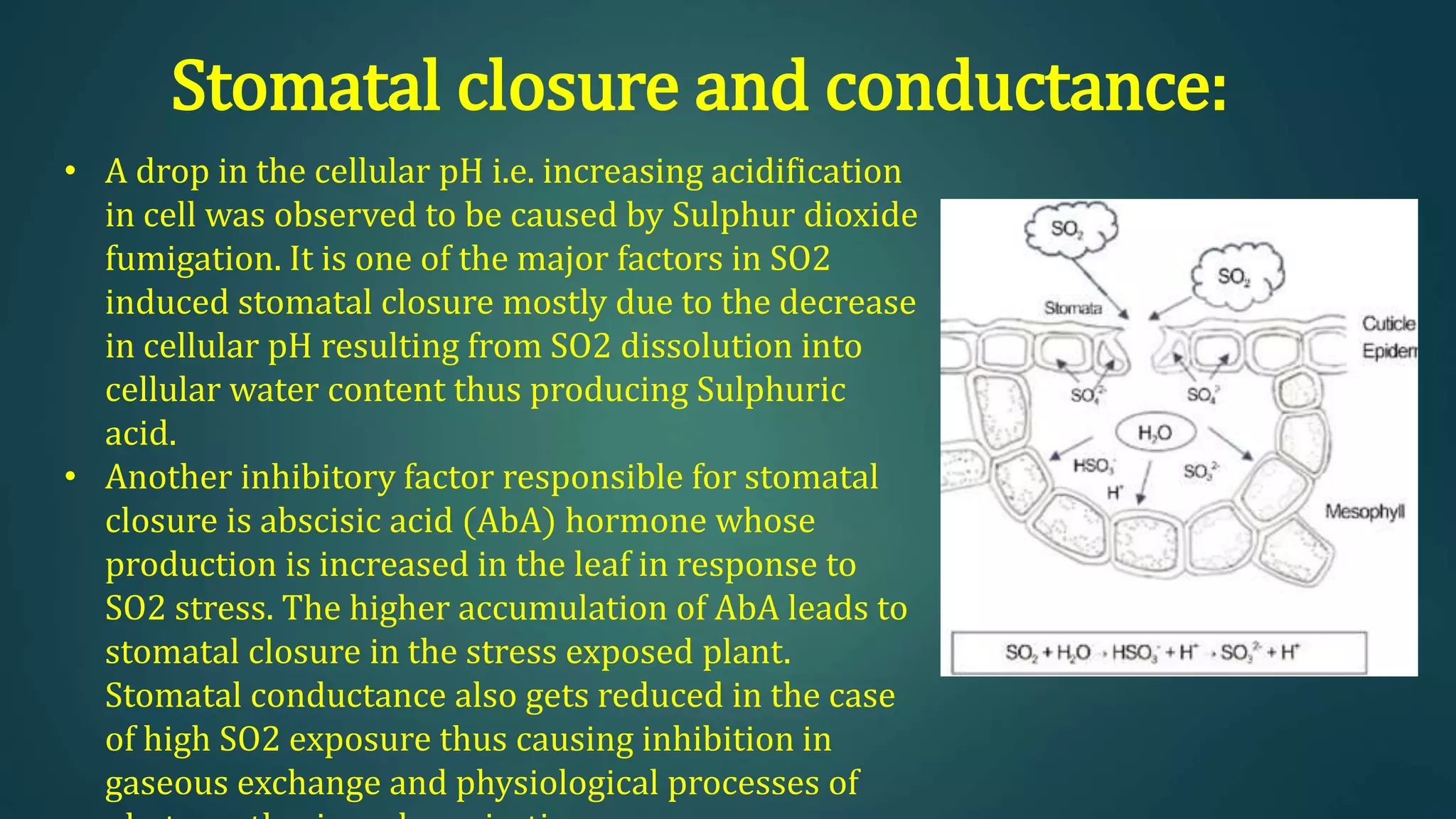
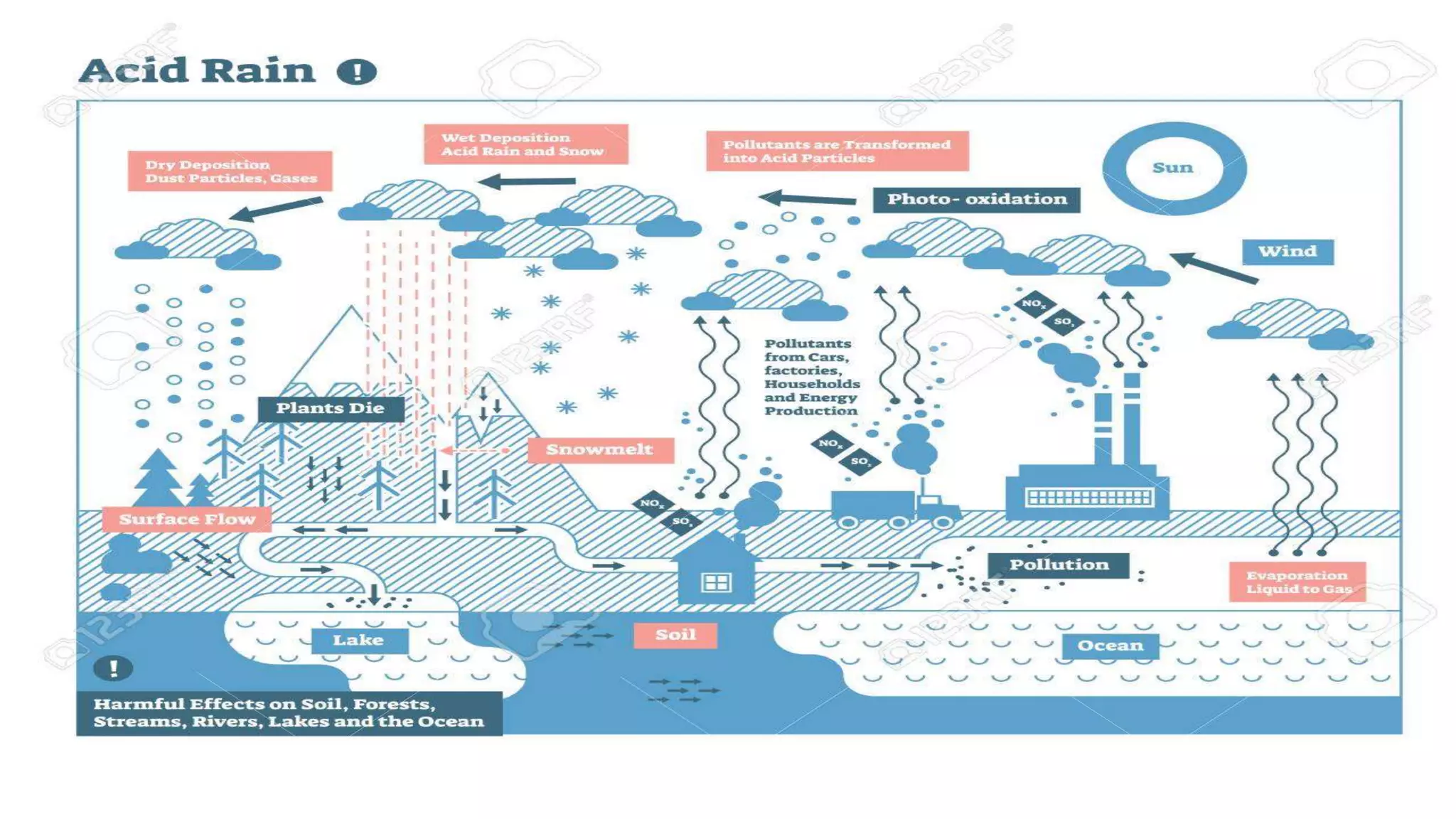
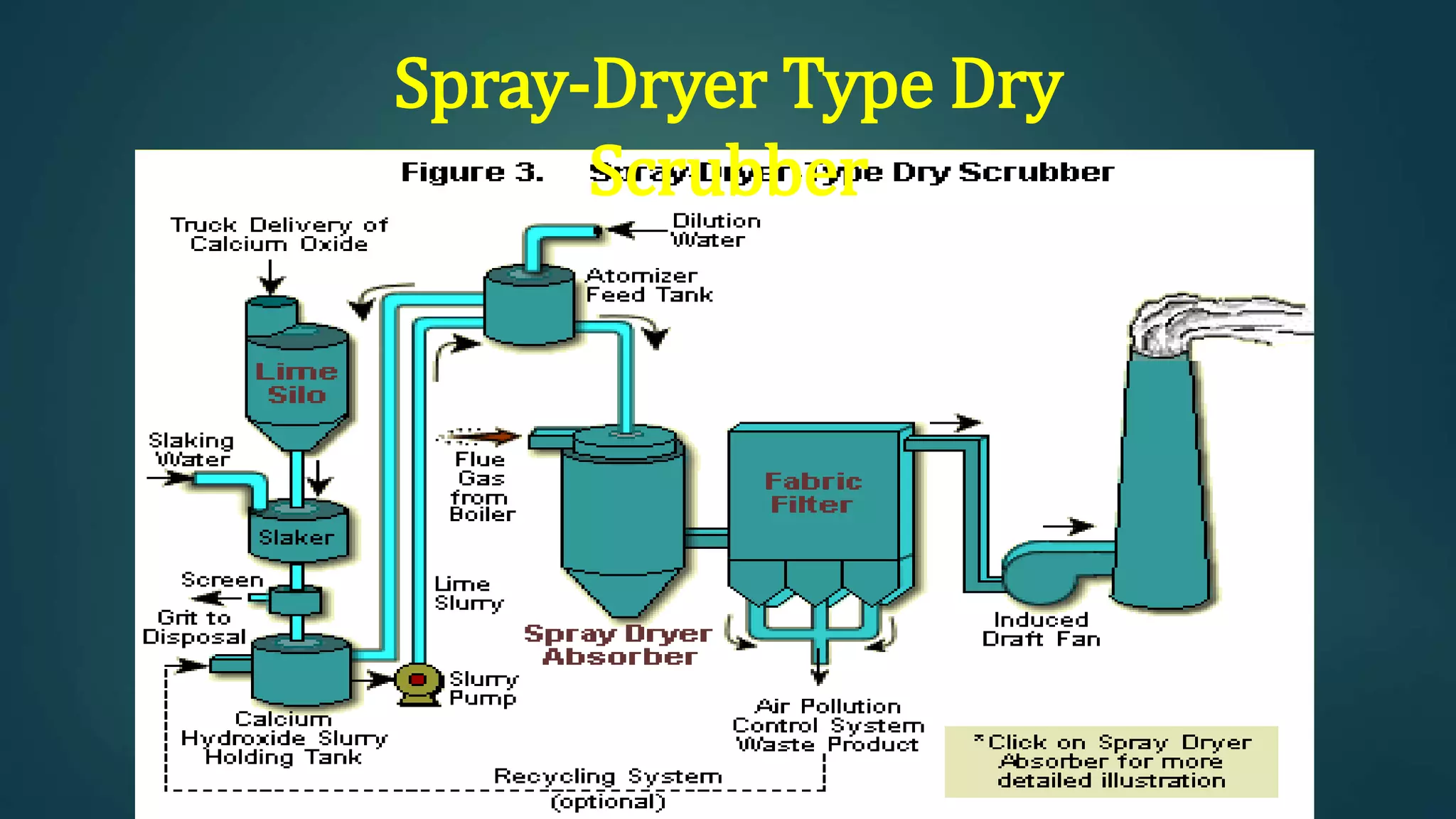
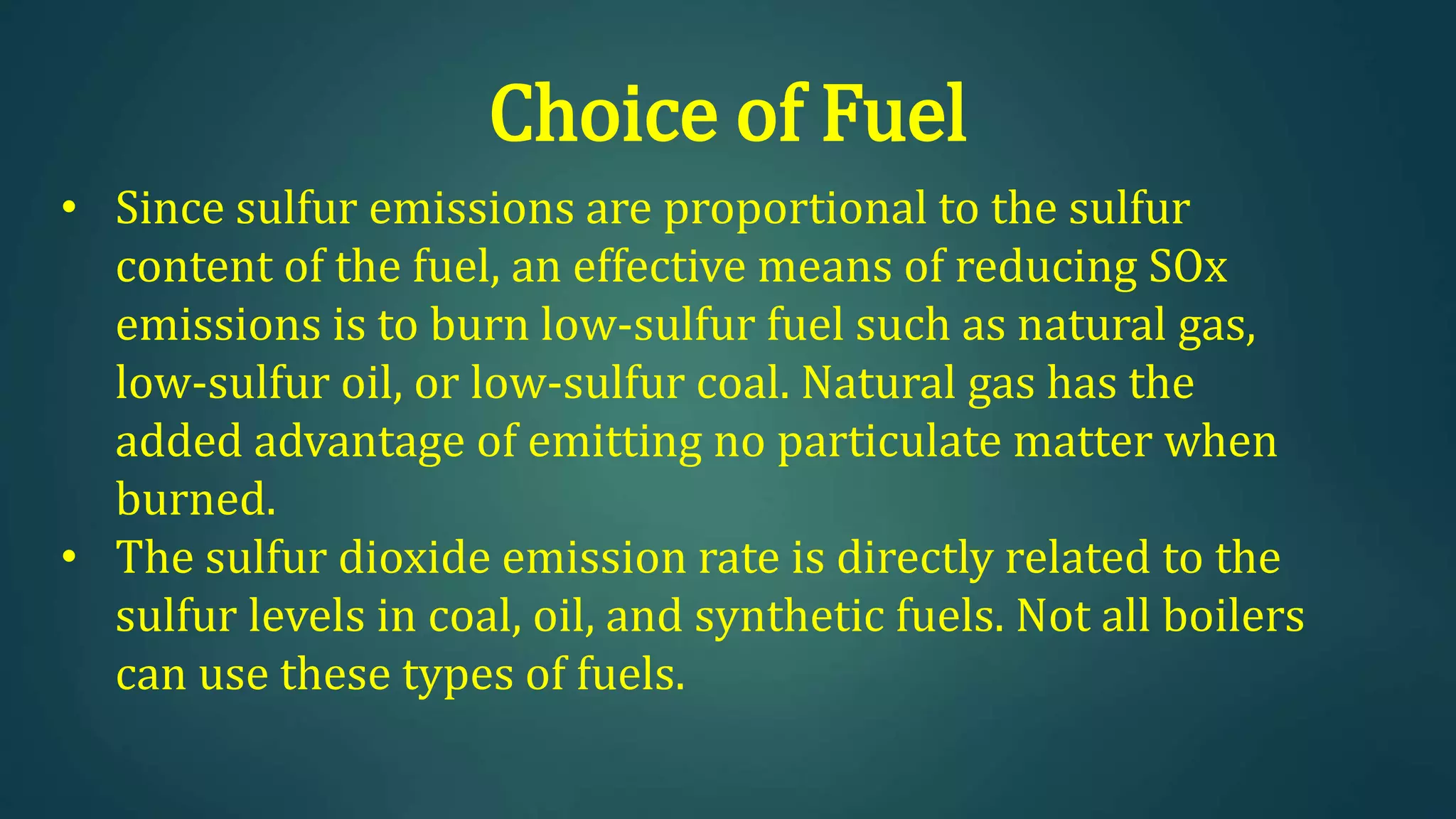
The document discusses sulfur dioxide (SO2), including its physical and chemical properties, sources of emission, and effects. It emits primarily from burning fossil fuels like coal. SO2 dissolves in water to form acid rain, which damages vegetation, property, and causes acidification of soils and lakes. Control techniques discussed include absorption using limestone scrubbers, spray dryer scrubbers, adsorption using lime, and using lower sulfur fuels.