1) The document discusses drug promotion and advertising standards, outlining that promotional information should be reliable, accurate, truthful, and capable of substantiation.
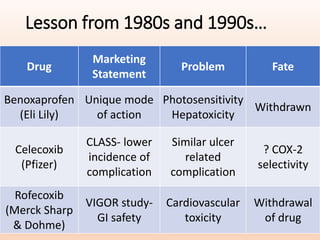
2) It provides examples of past drugs that were withdrawn or caused issues, such as benoxaprofen which was withdrawn due to hepatoxicity.
3) The document outlines the basic requirements for drug advertisements, including listing the brand name, active ingredients, approved uses, and safety information. It indicates reminders should include the brand name, generic name, and manufacturer information.