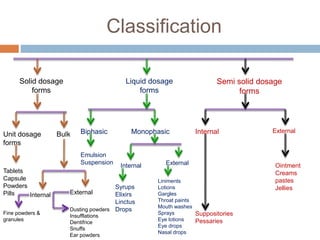
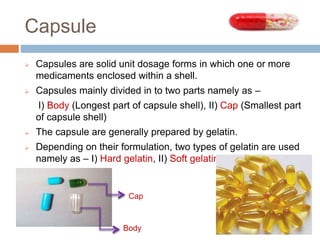
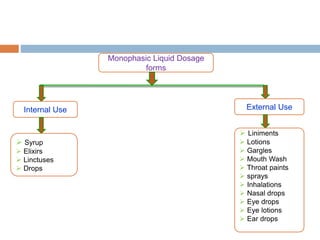
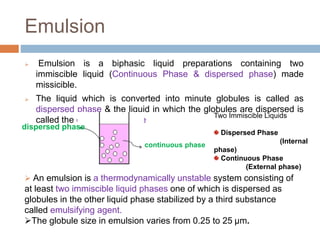
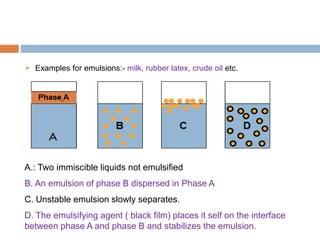
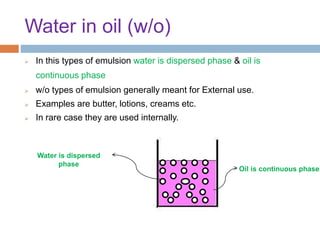
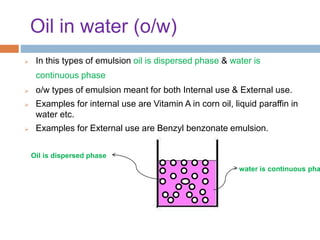
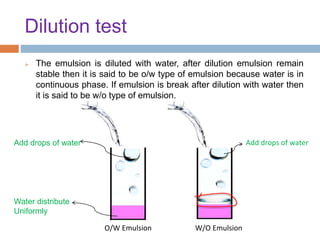
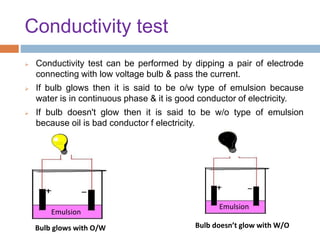
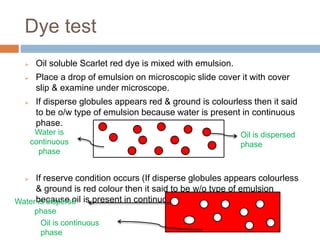
This document provides an introduction to different dosage forms. It defines dosage forms as combinations of drugs and excipients that deliver drug molecules to sites of action in the body. Dosage forms come in solid, liquid, and semi-solid forms and are classified based on their route of administration and drug release properties. The document discusses various types of solid dosage forms like tablets, capsules, and powders as well as liquid forms like solutions, suspensions, and emulsions. It provides examples of how dosage forms are tailored to meet specific drug delivery needs like sustained release or targeted delivery to tissues.