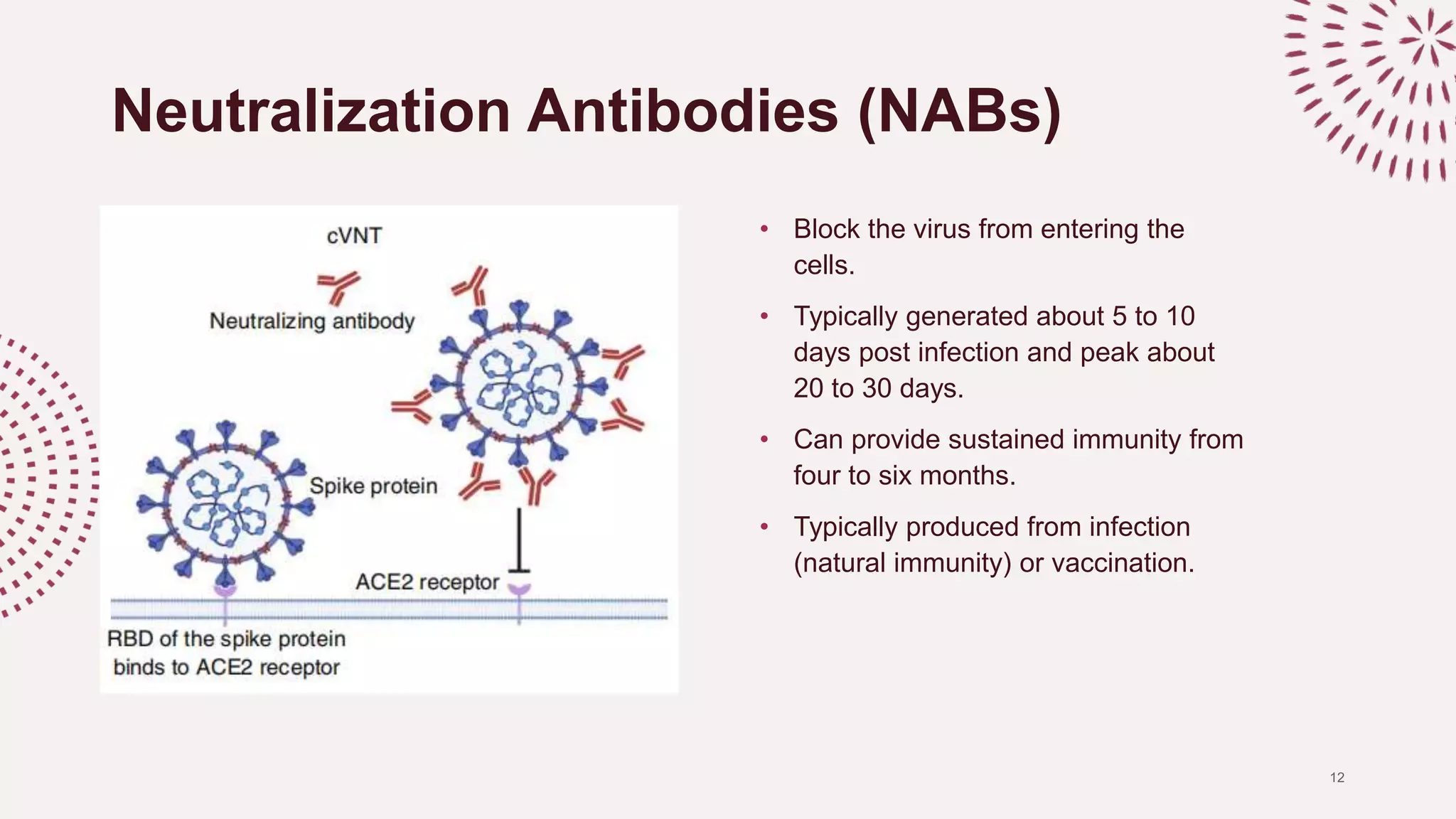
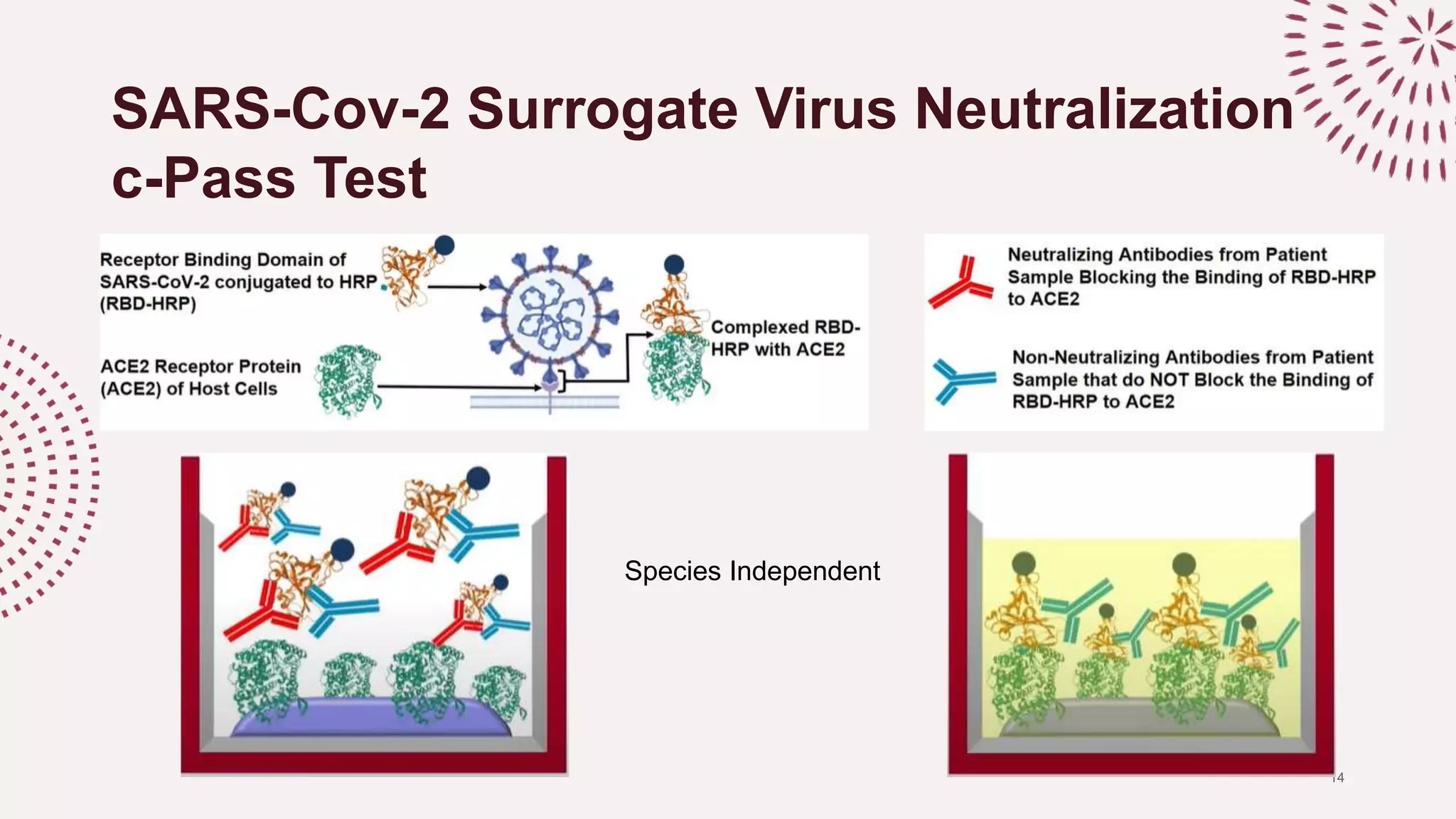
The document discusses the necessity of a robust serological test to detect neutralizing antibodies (NAbs) against SARS-CoV-2 to evaluate infection rates, herd immunity, and vaccine efficacy. It explains various testing methods including molecular and serological tests, highlighting the advantages of the c-pass surrogate virus neutralization test (SVNT) which is easy, safe, and rapid. The SVNT demonstrates strong specificity and sensitivity for detecting NAbs, making it useful for a wide range of COVID-19 investigations.