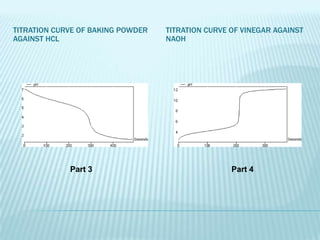
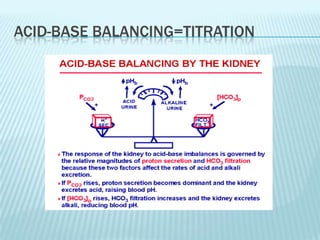
The document describes an experiment using a data logger to perform acid-base titrations. The objectives are to construct titration curves, learn principles of acid-base titrations using the data logger as an educational tool, and determine concentrations of analytes like acetic acid in vinegar and sodium bicarbonate in baking powder. The procedure involves titrating samples like HCl, NaOH, baking powder, and vinegar against each other to generate titration curves and determine concentrations. The results show various titration curves. Maintaining acid-base balance through systems like buffering and respiration is important for health.