The document discusses dampening solutions used in offset printing. It provides information on:
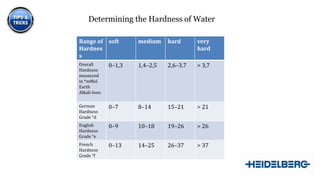
1) The ideal properties of a dampening solution including a water hardness of 8-12°dH and a pH balance of 4.8-5.5.
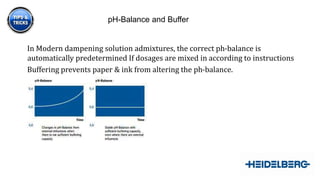
2) The various components of water that affect its quality for use in dampening solutions and how additives can help adjust the pH balance and prevent issues.
3) How to test the alcohol content of dampening solutions using an areometer to measure volume and temperature, which impact conductivity.
4) The importance of wetting plates adequately and how alcohol is commonly used as a wetting agent to lower surface tension despite its evaporation effects.