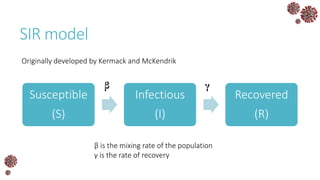
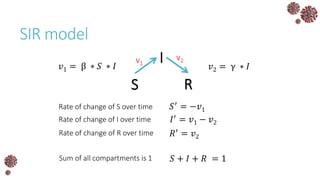
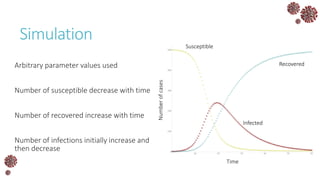
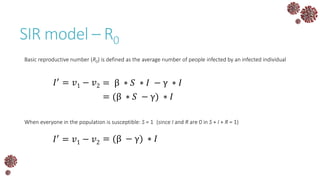
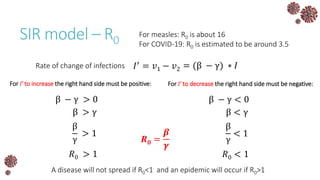
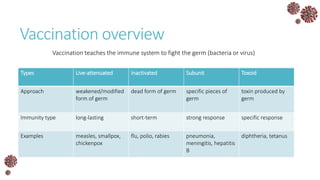
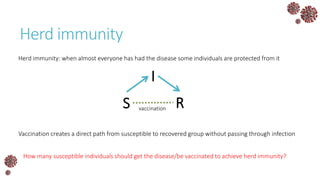
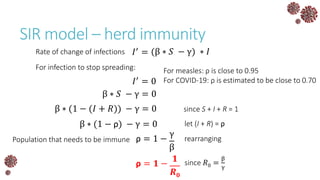
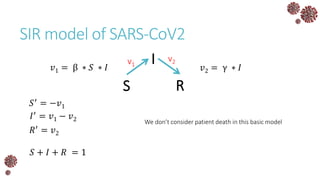
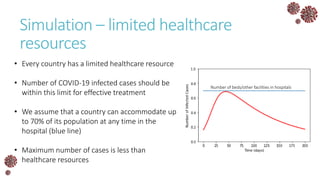
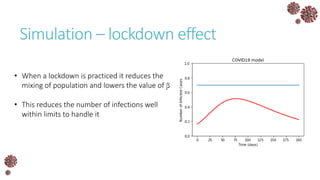
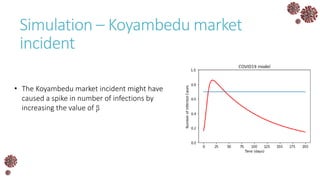
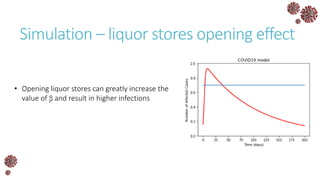
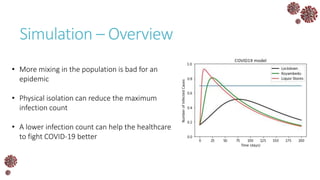
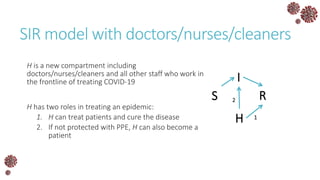
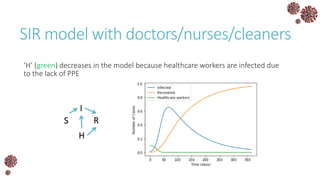
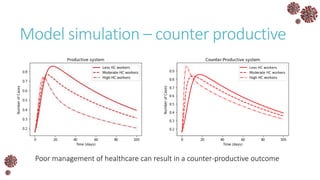
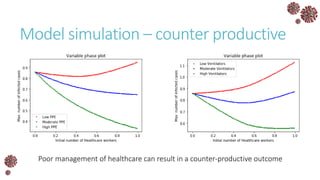
The document discusses the mathematical modeling of epidemics, with a focus on COVID-19, explaining historical modeling efforts and key concepts like the SIR model. It highlights the importance of herd immunity, vaccination strategies, and the impact of healthcare resource limitations on disease spread. The document also explores modifications of models to account for factors like healthcare workers and the effects of lockdowns and social behaviors on infection rates.