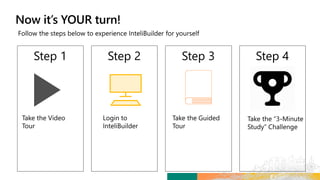
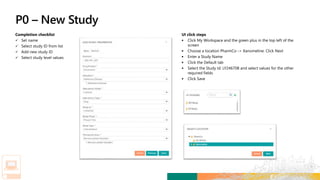
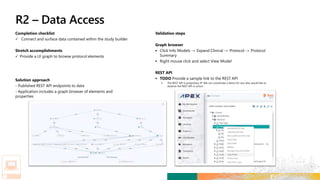
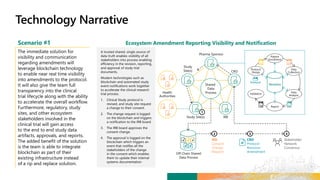
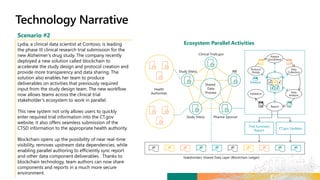
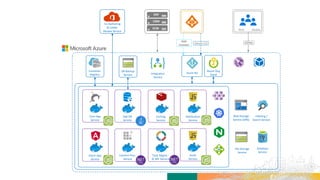
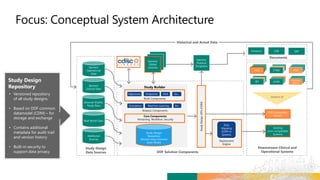
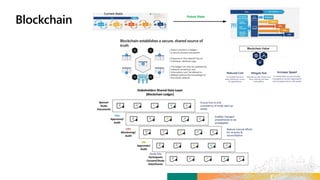
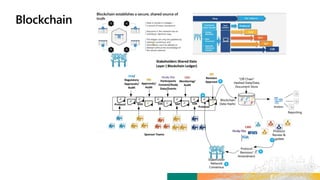
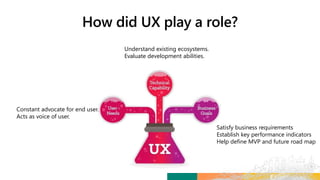
TransCelerate held a hackathon to demonstrate a digital clinical study design platform that can build a global clinical study protocol in 3 minutes. The agenda included an interactive demonstration of the platform and user guides. The platform uses blockchain technology to provide transparency and visibility into the clinical trial process for all stakeholders. This allows for near real-time sharing of study protocols, amendments, and reports to accelerate drug development.


























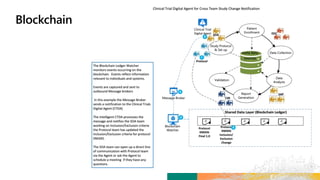











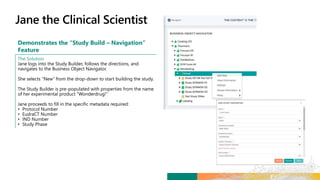


![Demonstrates the “Initiate Study Build & Study
Build Design”
The Solution
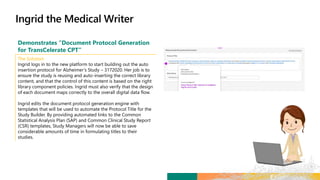
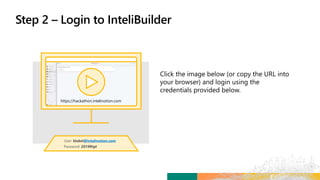
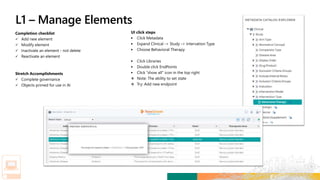
Matteo logs in to the new platform to retrieve Alzheimer’s Study
– 3172020 and initiate the Study Design process.
Matteo builds the Protocol Title of the study based on hyperlinks
to prepopulated libraries and templates. He then defines the
Study Objective as follows: “To assess the effect of [study
intervention #1] on the ADAS-COG and CIBIC + scores at Week
[X] in participants with Mild to Moderate Alzheimer’s Disease.”
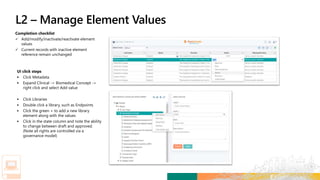
Matteo next enters the Study Masking Information,
Intervention, and Arms. For these categories, he chooses
Double Blind, Xanomeline, and High Dose respectively.
Matteo can select the Objectives and Endpoints for the study
using the standards managed and governed in the Objectives and
Endpoints libraries. Matteo selects the appropriate Objectives,
Objective Level and related Endpoints. For each Endpoint, the
appropriate visit Timeframes, Units and Biomedical Concepts are
selected from controlled standard metadata terms.](https://image.slidesharecdn.com/transceleratehackathon04192020fornn-200420140341/85/Transcelerate-hackathon-04192020_for-nn-70-320.jpg)