TransCelerate is a nonprofit organization that aims to accelerate medical research by improving collaboration across the pharmaceutical industry. It has developed a Common Protocol Template (CPT) to standardize clinical trial protocols. The CPT provides a streamlined template for protocol content and format to make protocols easier to interpret, reduce complexity and costs, and enable automation. The CPT benefits various stakeholders by improving efficiency and quality. Its adoption by sponsors is valuable as it leverages industry expertise, supports compliance, and balances quality improvements with efficiency gains over time.




![Copyright ©2015 TransCelerate BioPharma Inc., All rights reserved. 6
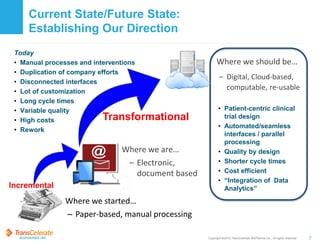
• A common protocol template structure
with proposed model language
• Streamlined content enables
identification of critical information for end
users
• Begin working towards common endpoint
definitions to align with Clinical Data
Interchange Standards Consortium
(CDISC) Therapeutic Area (TA) data
standards. Asthma and Diabetes
available in the first release.
• Reconnect processes (protocol, eCRF,
development)
• Transformation of the design process
o Analytics-driven trial design, modelling,
scenario planning
• Role-based access to protocol
information (Principal Investigator [PI],
Ethics, Participants)
• Connection to other systems
Common Protocol Template is Intended to
Prepare for the Future State
Human-
Readable
Protocol
IRB/IECs
Sites
Regulators
Foundation
Machine-
Readable
Protocol Metadata driven
processes
Content Reuse
Disclosure
SAP
CSR
eCRF
Statistical Output
Future](https://image.slidesharecdn.com/cptimpltk-exec-summaryv002-170706164136/85/Common-Protocol-Template-Executive-Summary-6-320.jpg)