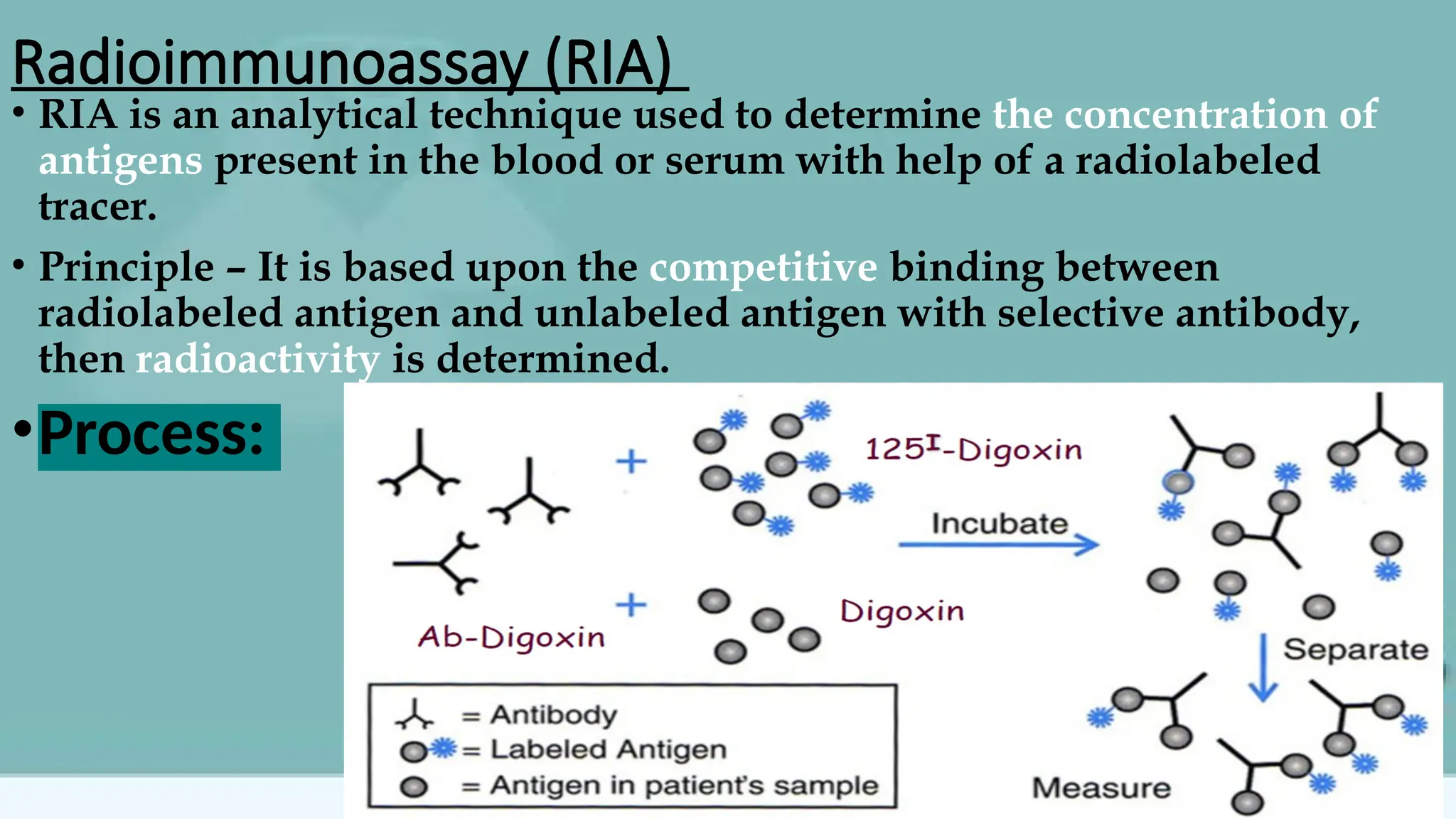
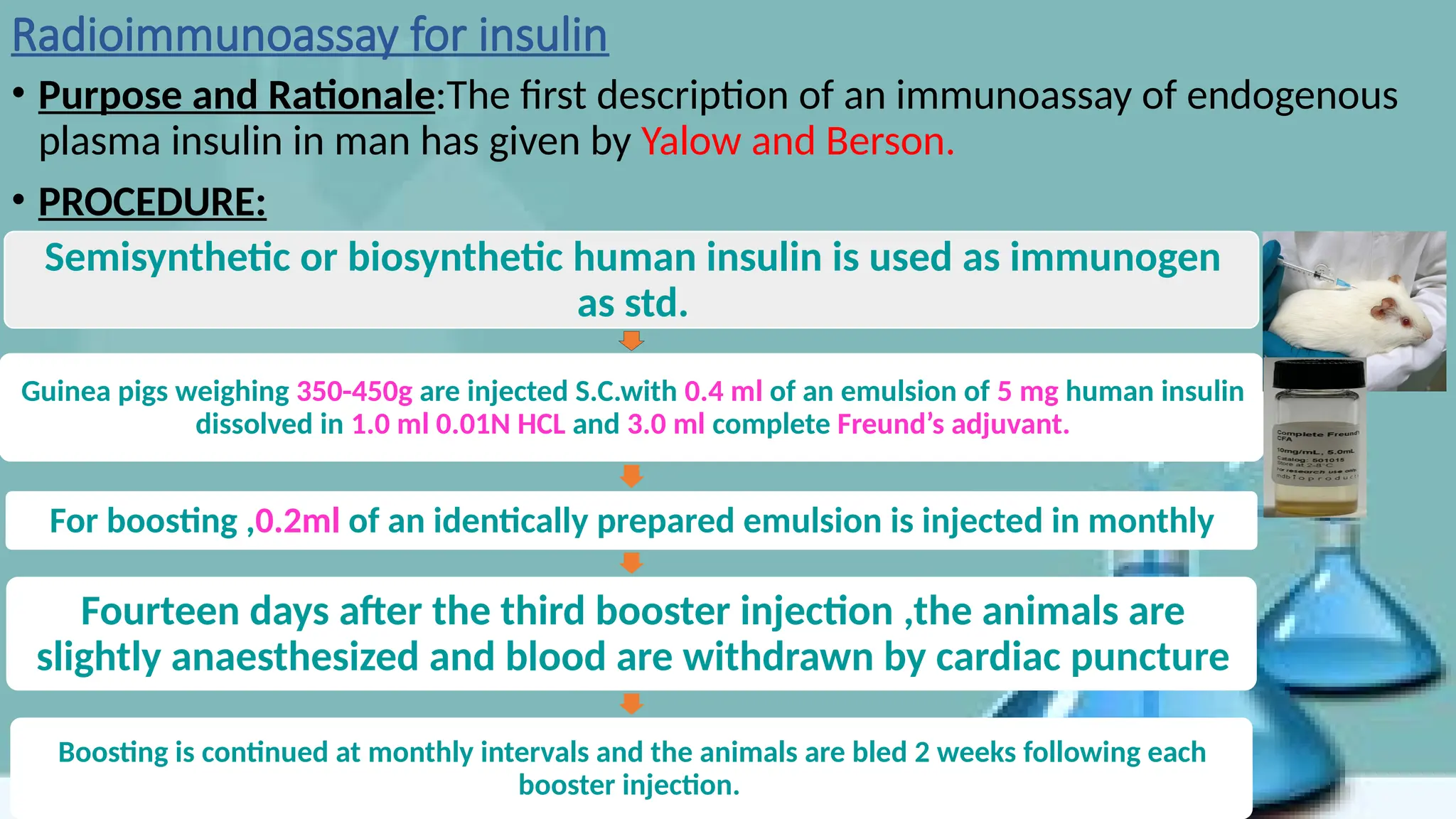
The document provides an overview of immunoassays for digoxin and insulin, detailing the principles, methodologies, and importance of these assays in monitoring cardiac glycoside and hormone levels. It explains the underlying mechanisms of immunoassays, types of antibodies, antigens, and various analytical methods like radioimmunoassay and enzyme immunoassay. Additionally, it highlights the clinical relevance of measuring digoxin concentrations due to its narrow therapeutic range and the role of insulin in blood glucose regulation.