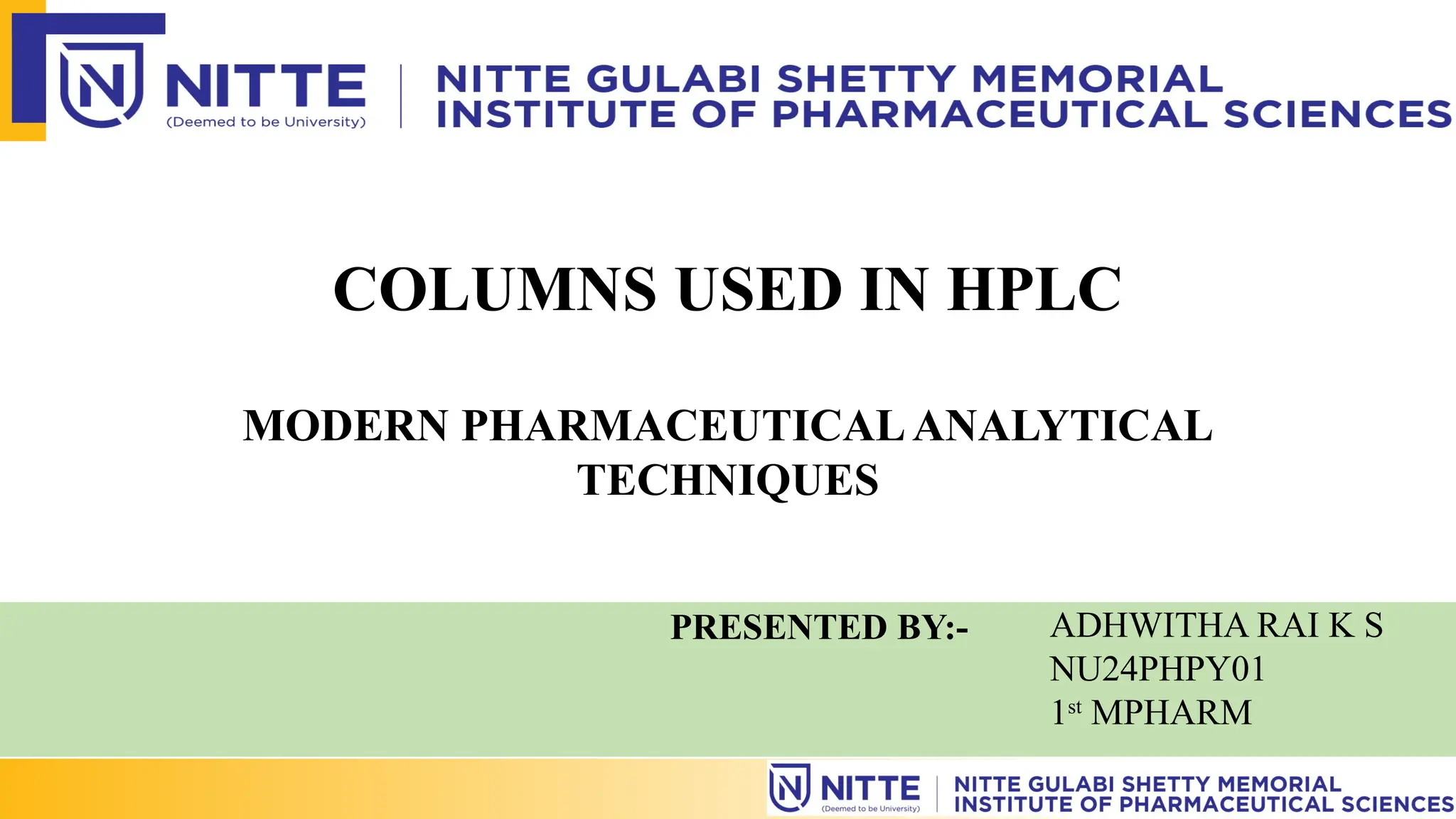
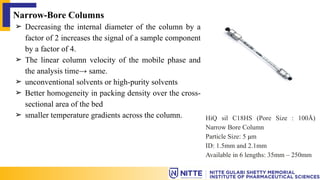
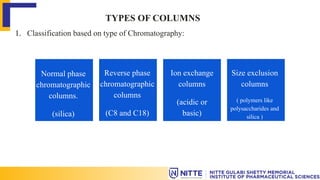
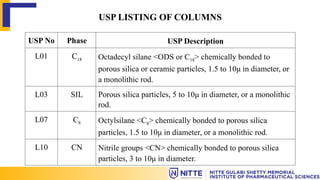
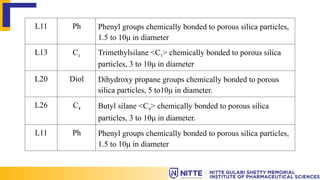
The document discusses the various types of columns used in High-Performance Liquid Chromatography (HPLC), including separation, analytical, and guard columns, as well as their construction, packing materials, and factors affecting selection. It details the importance of column temperature, dimensions, and types of chromatography such as reverse phase, ion exchange, and size exclusion. The paper emphasizes that the choice of column is critical for effective separation of components in mixtures and provides a USP listing for different columns.