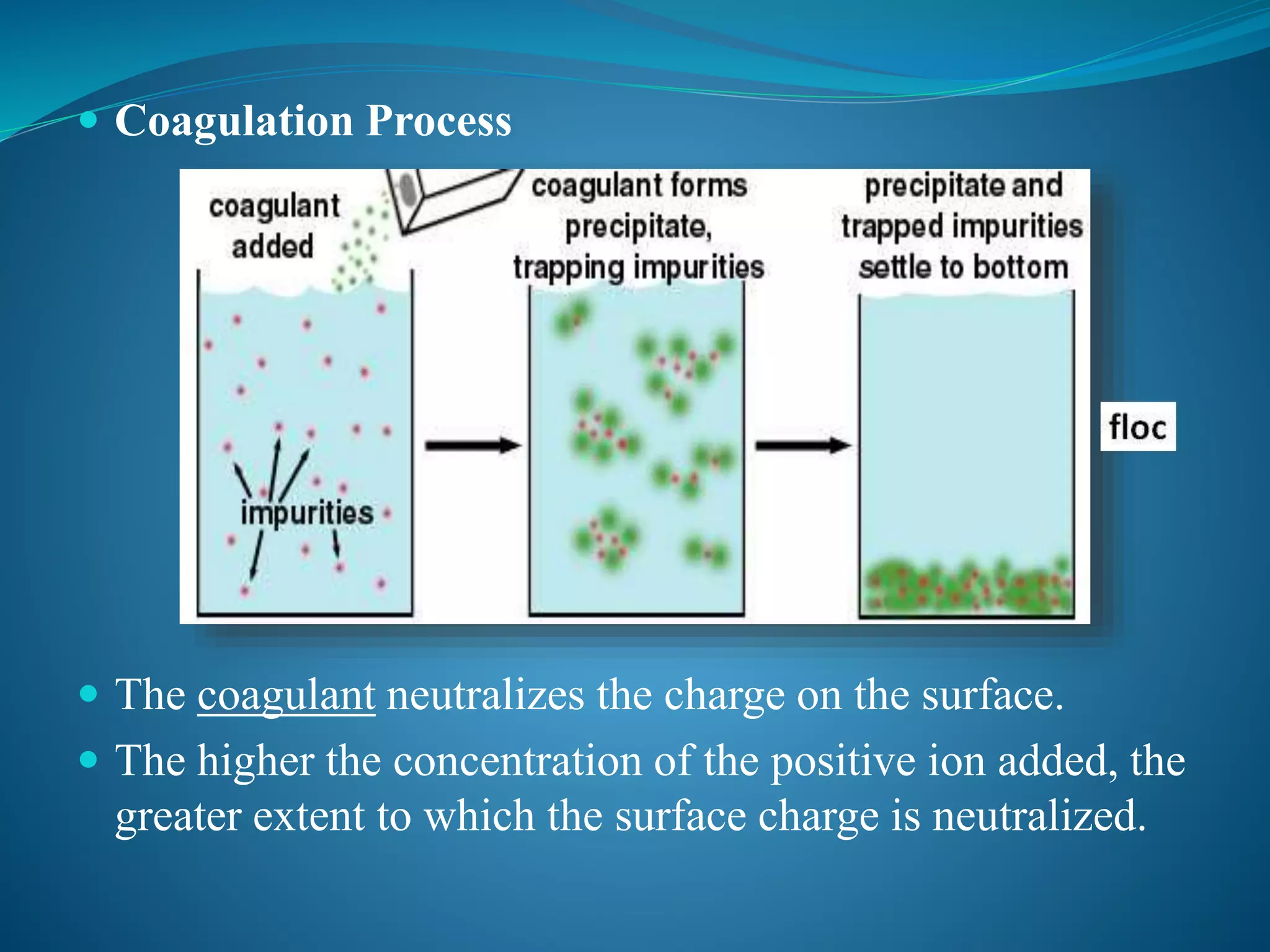
This document discusses the water treatment process of coagulation. Coagulation involves adding chemicals called coagulants to water to neutralize surface charges on particles and allow them to stick together and form larger particles that settle out via gravity. Key coagulants discussed are aluminum sulfate and ferric sulfate. The document also covers colloid stability, the coagulation process, properties of effective coagulants, and coagulant aids which add density and strength to the settling floc particles.