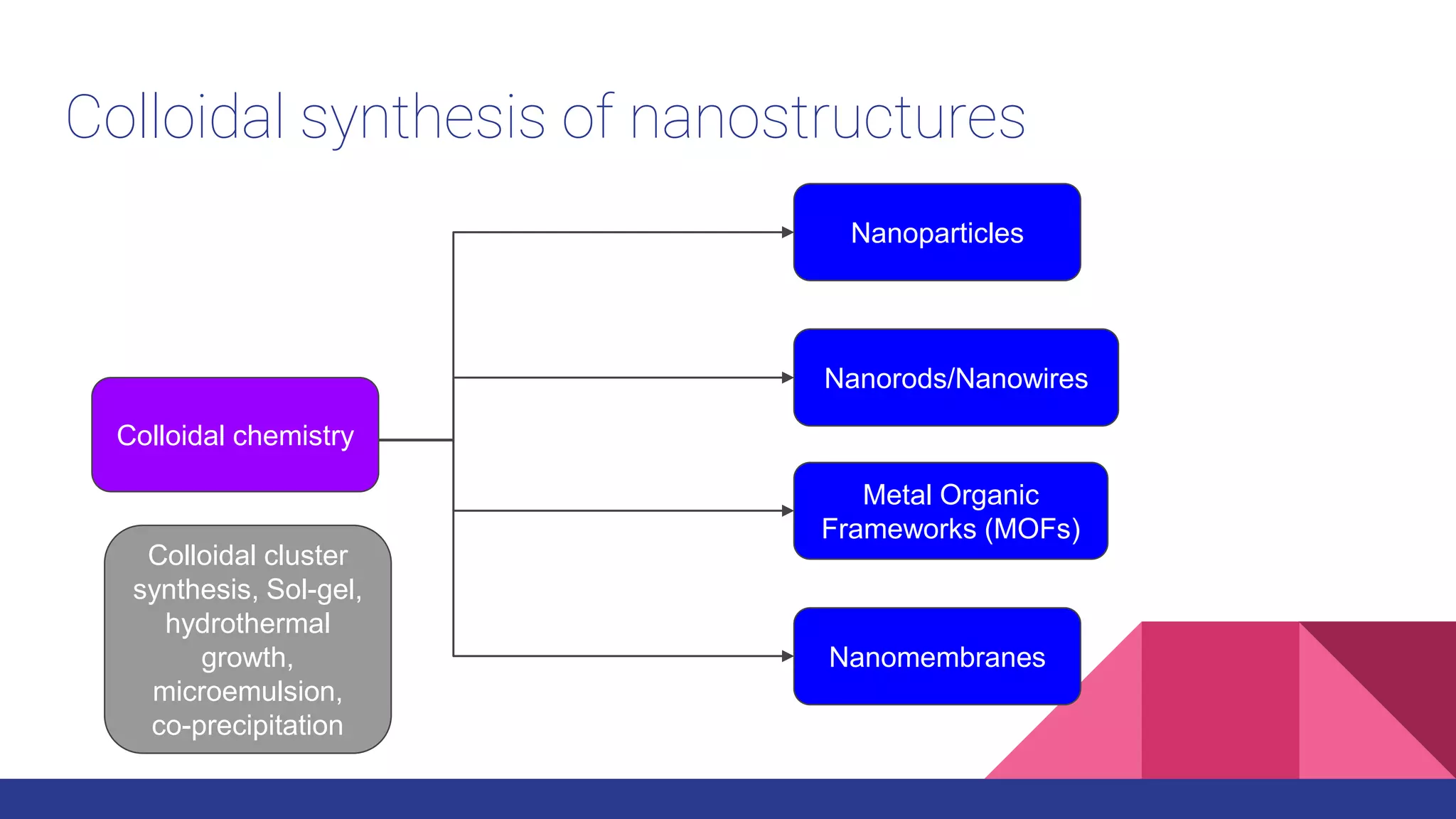
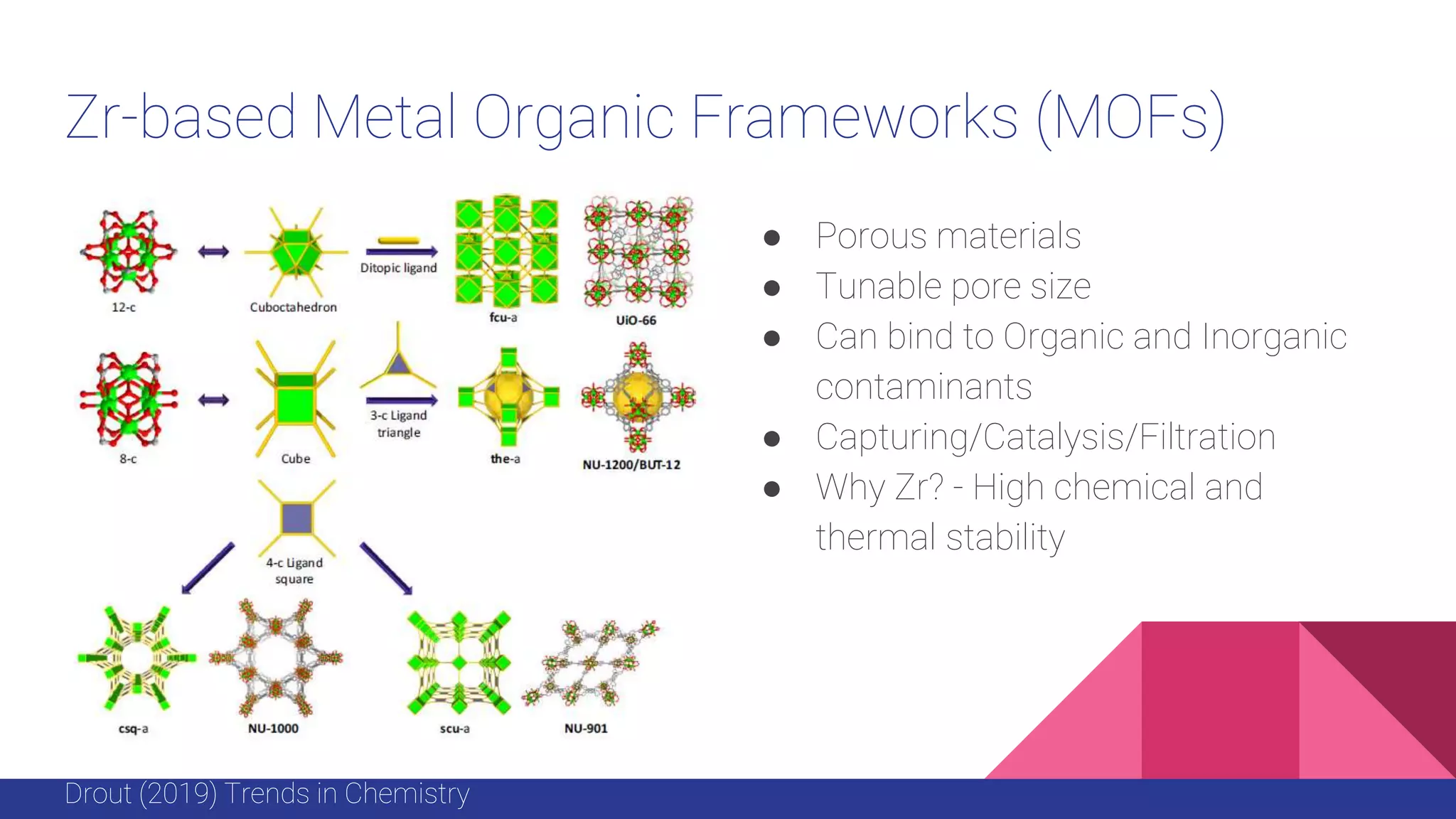
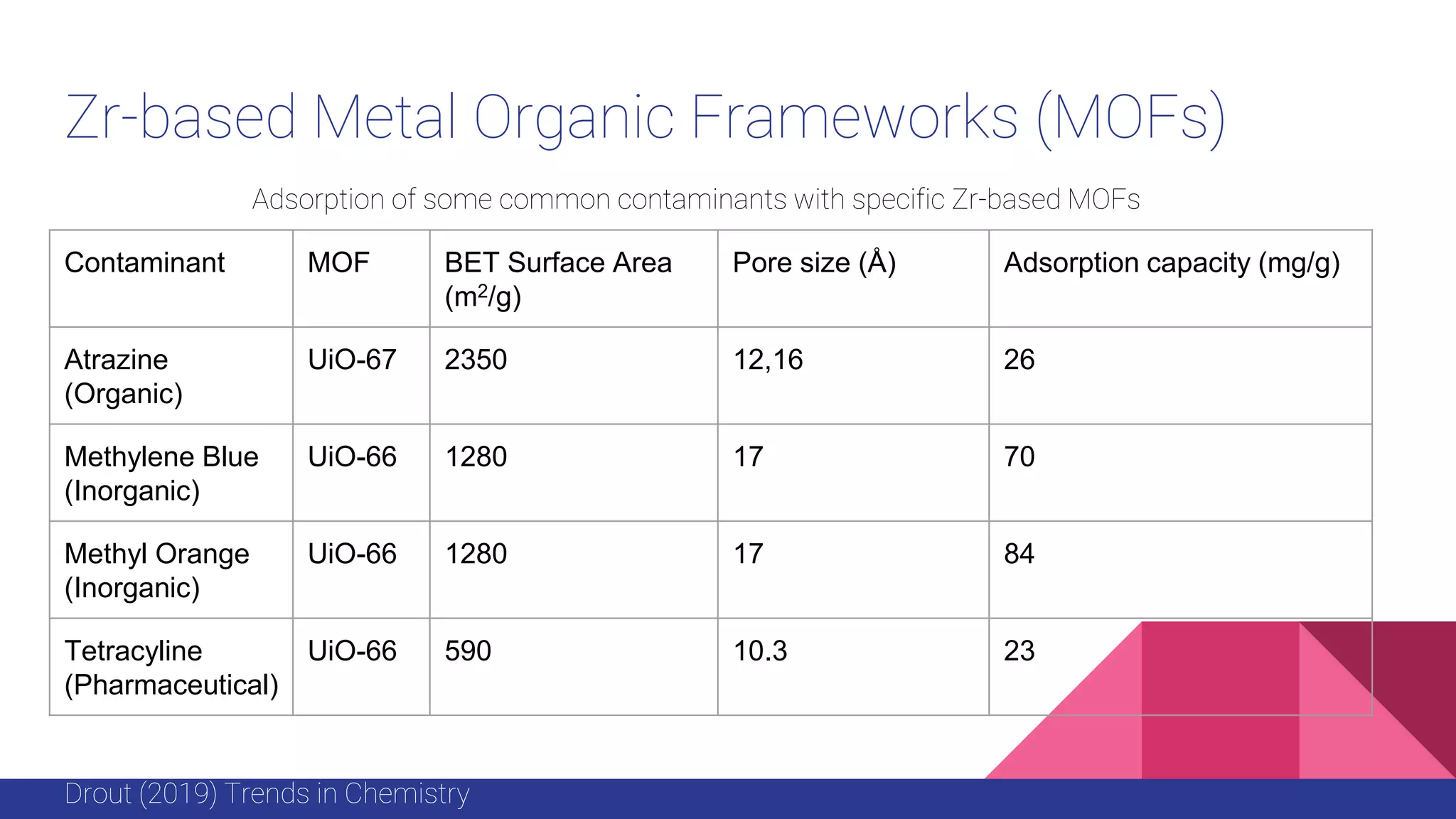
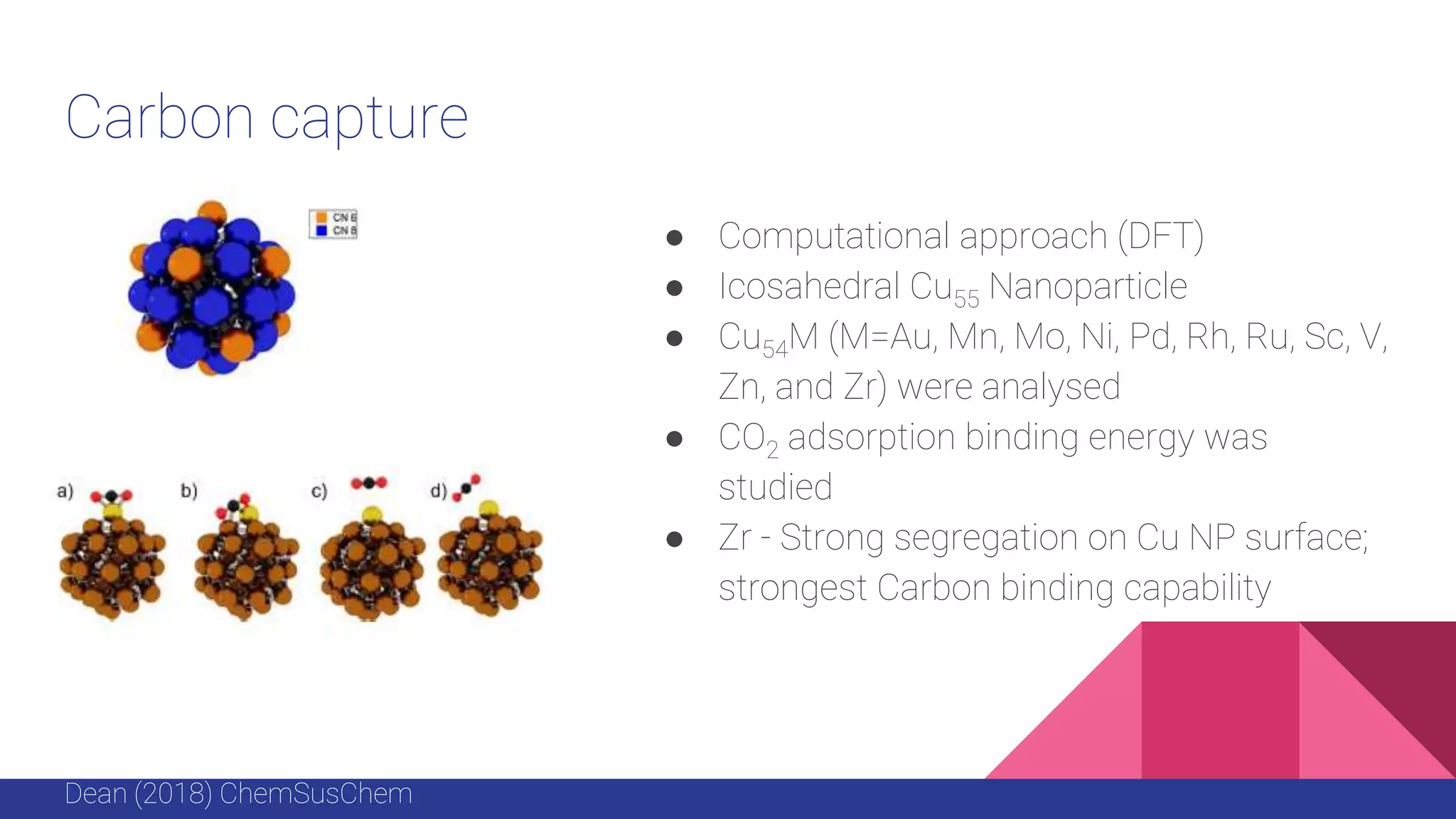
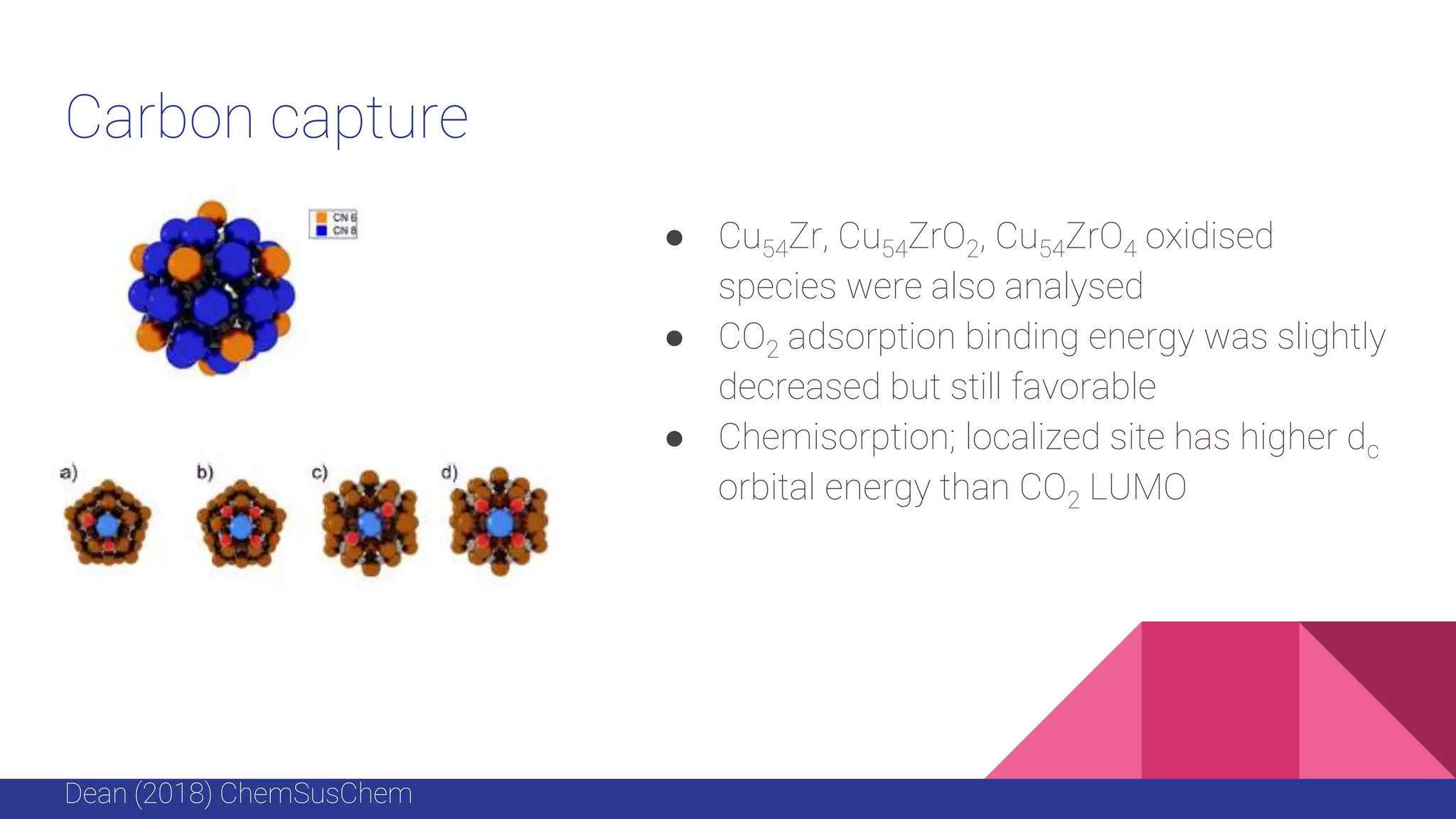
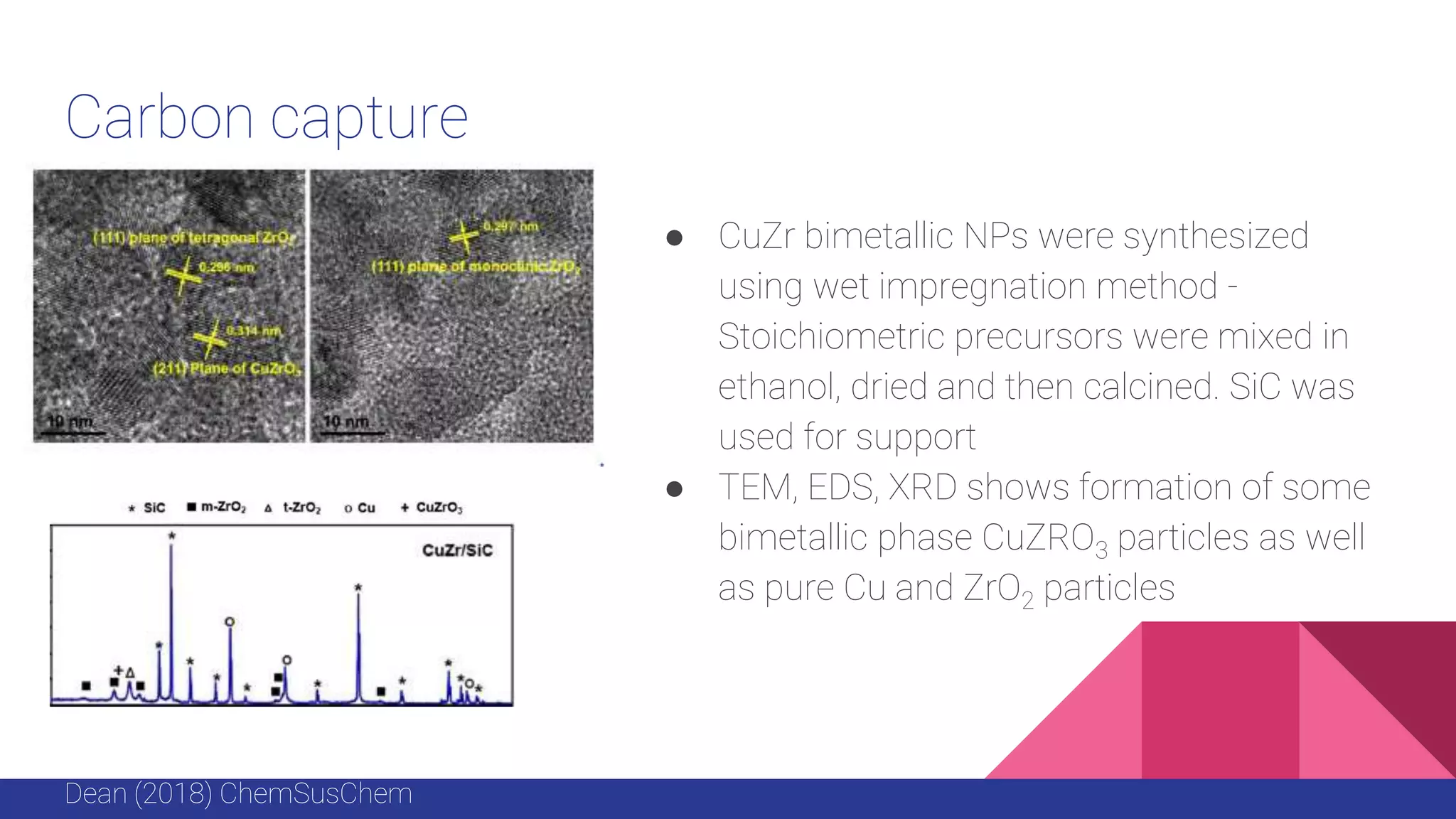
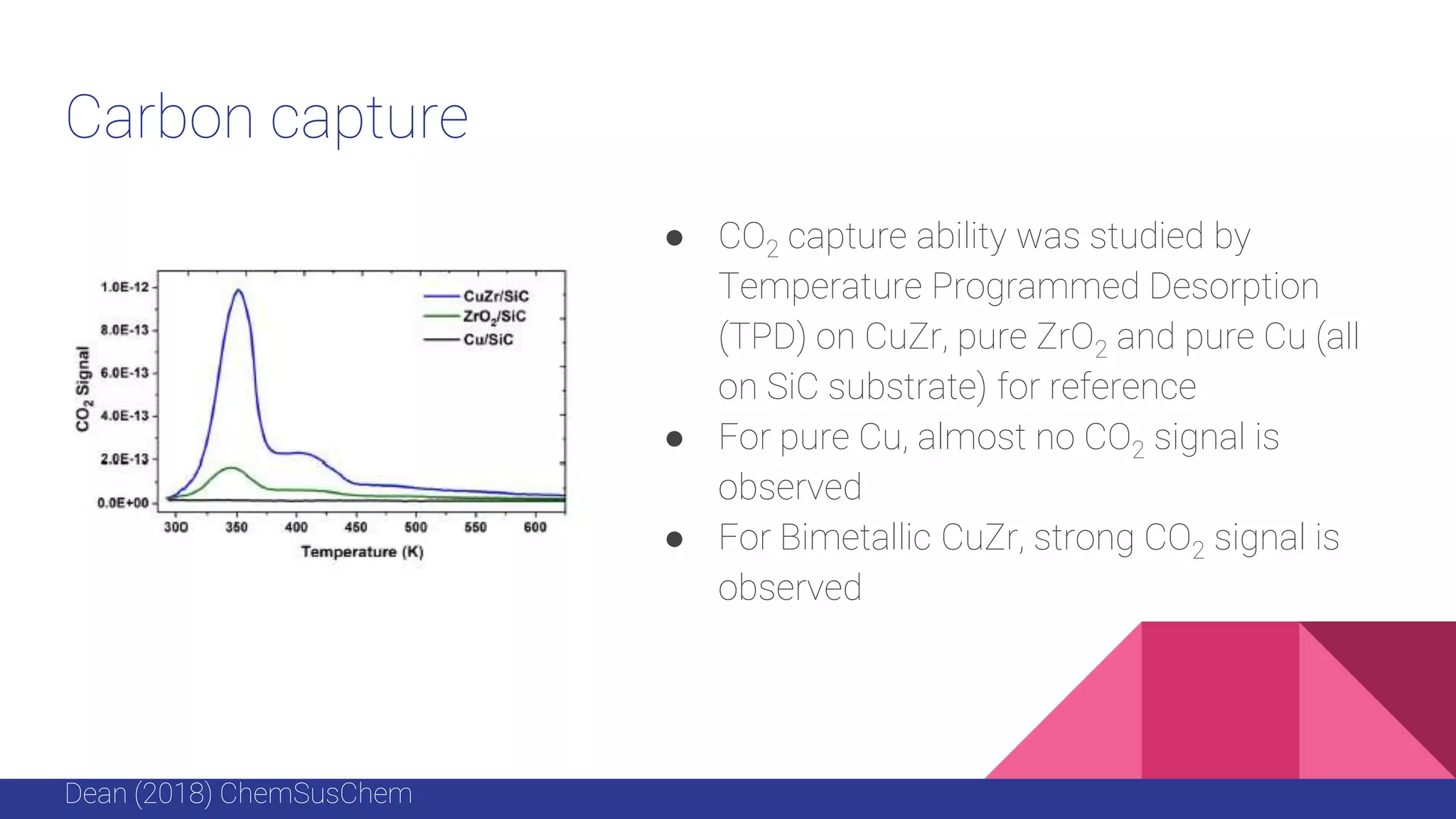
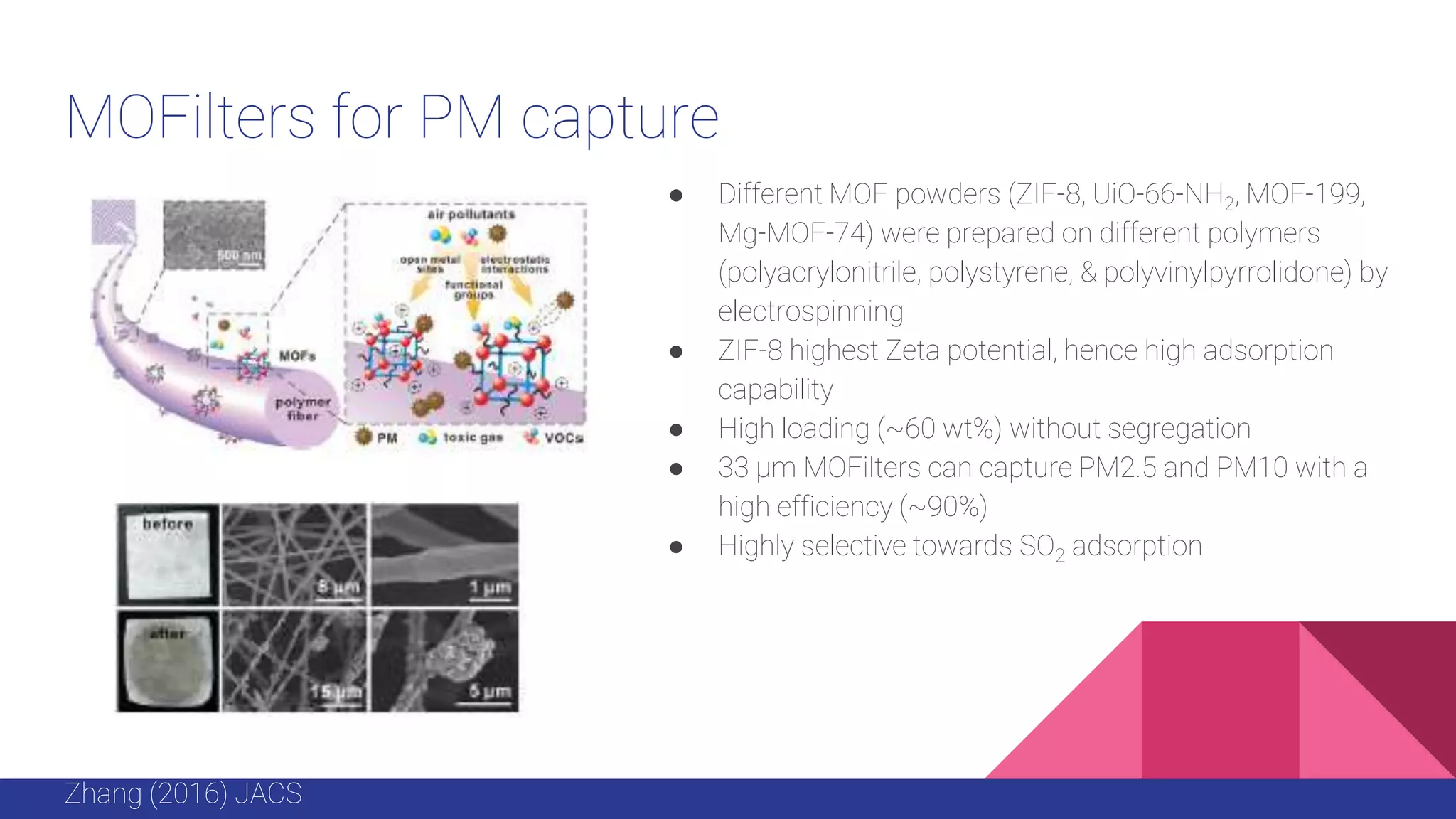
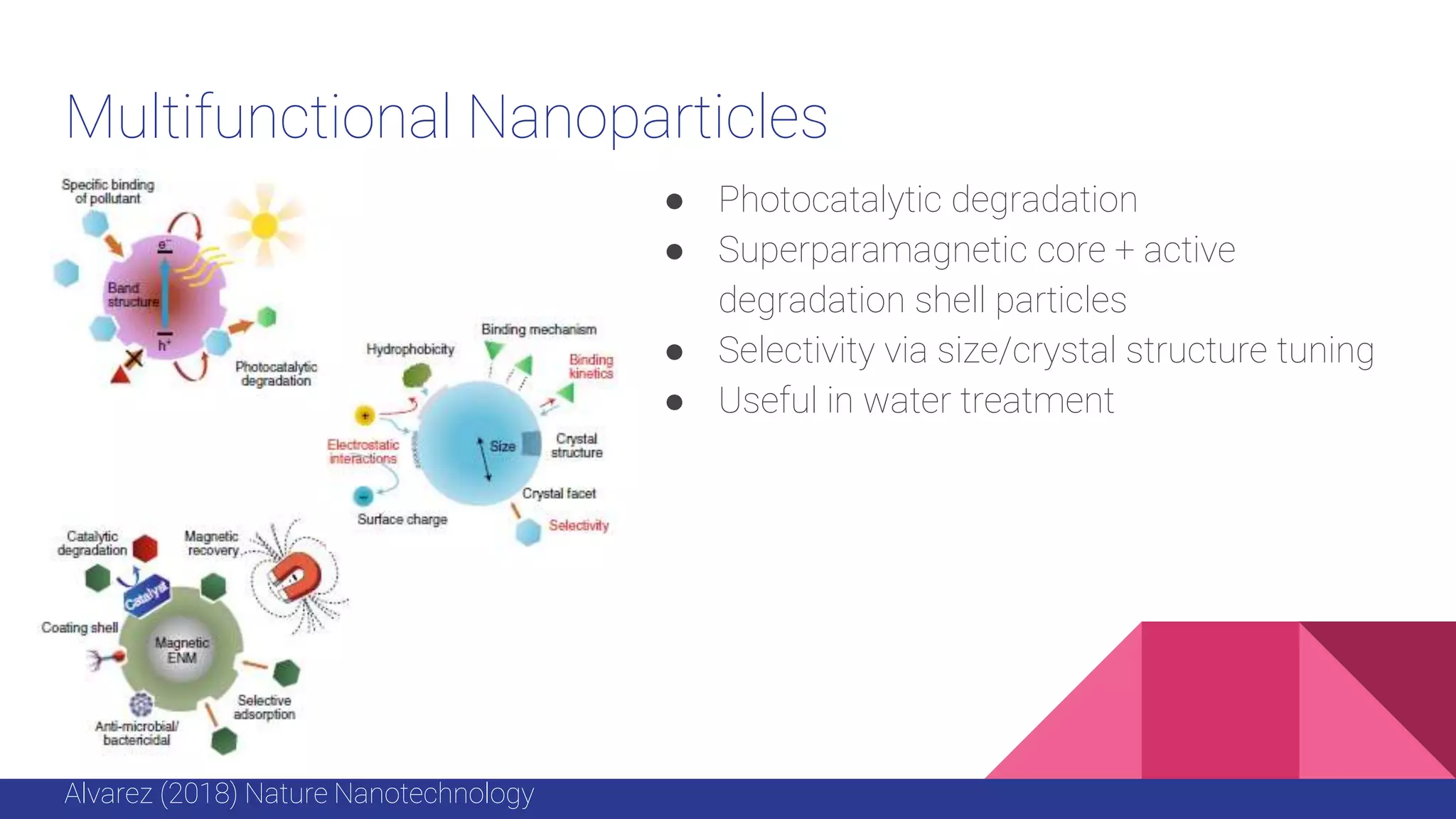
This document discusses the use of colloidal nanostructures for environmental contaminant capture. It provides two case studies: (1) Zr-based metal organic frameworks (MOFs) that have high surface areas and pore sizes allowing them to effectively adsorb various organic and inorganic contaminants; and (2) CuZr bimetallic nanoparticles that show favorable binding of carbon dioxide through computational modeling and experiments. The document also briefly mentions other applications of multifunctional nanoparticles for water treatment and challenges in scaling up these colloidal structures.