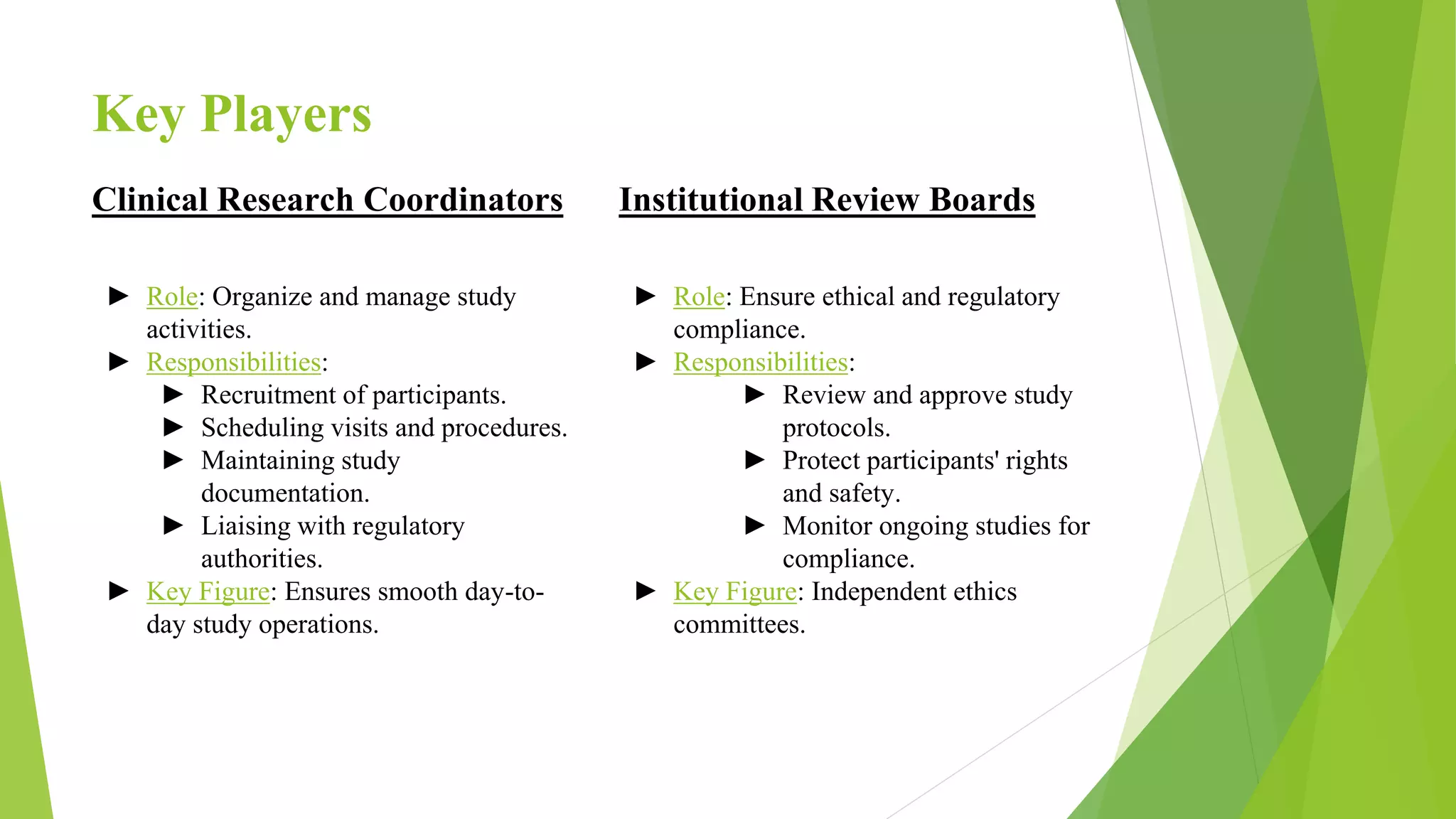
Clinical research involves systematically investigating new medical treatments and therapies through clinical trials on human volunteers. It is vital for advancing medical knowledge, improving patient care, and ensuring safety. Clinical trials progress through four phases from initial safety testing to large-scale effectiveness trials. Researchers must adhere to strict ethical and regulatory guidelines to protect participants and ensure valid results. While challenging, clinical research benefits patients through medical advancements and improved care.