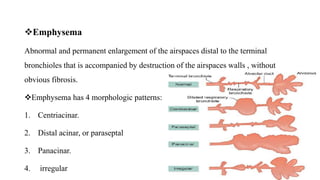
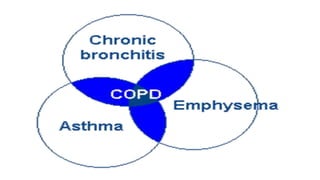
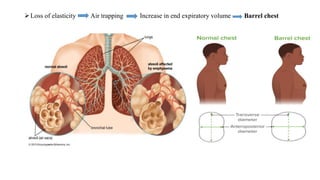
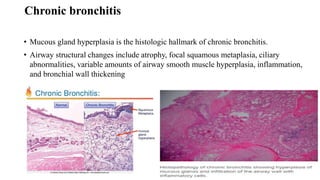
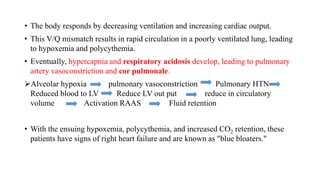
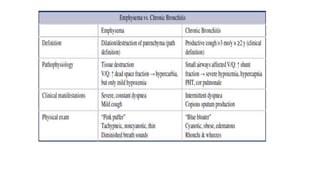
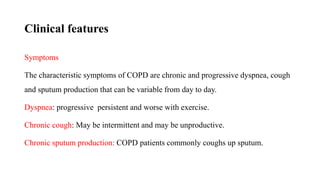
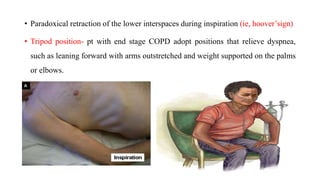
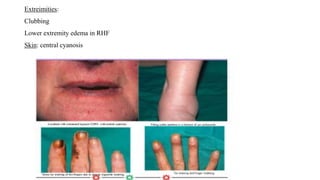
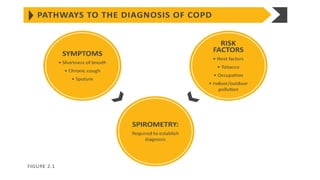
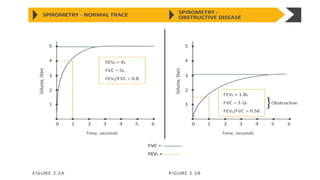
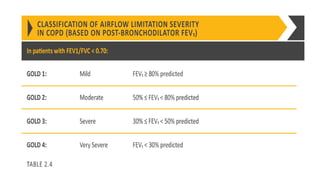
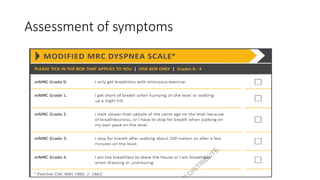
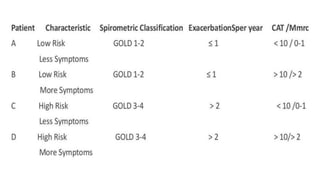
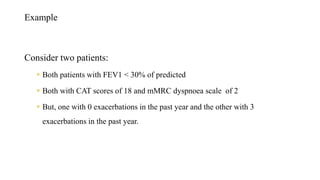
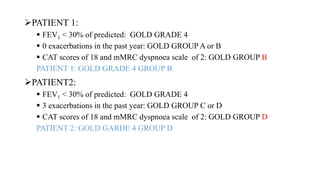
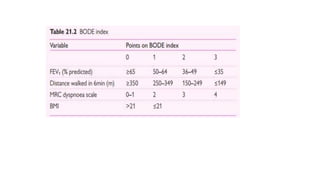
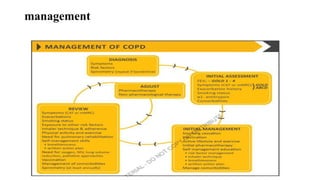
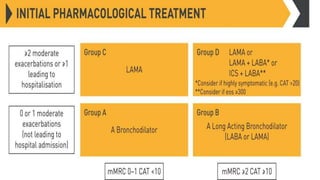
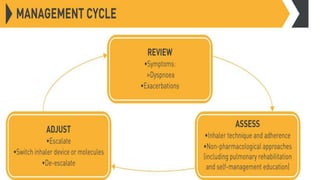
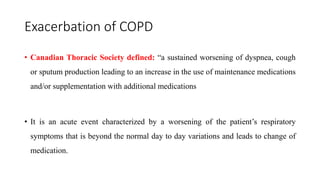
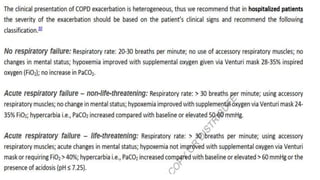
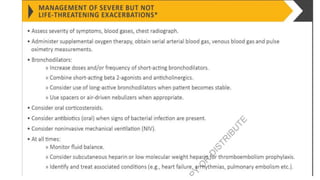
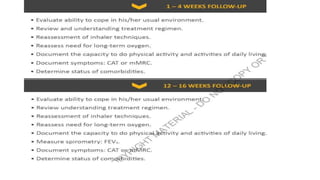
The document provides a comprehensive overview of chronic obstructive pulmonary disease (COPD), detailing its definition, epidemiology, pathophysiology, symptoms, diagnosis, and management strategies. It emphasizes the importance of smoking cessation, pulmonary rehabilitation, and the management of exacerbations, while also highlighting the economic burden and public health challenge posed by COPD. Additionally, the document discusses the association of comorbidities and conditions such as asthma-COPD overlap syndrome (ACOS) in the clinical context of COPD.