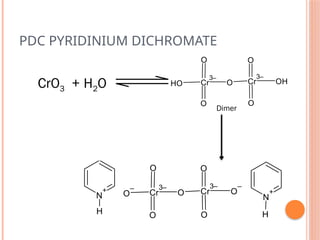
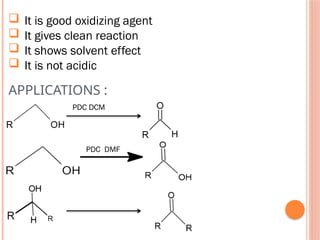
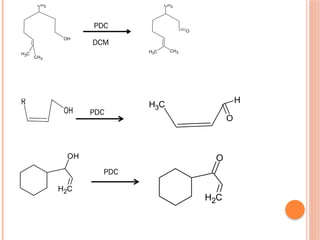
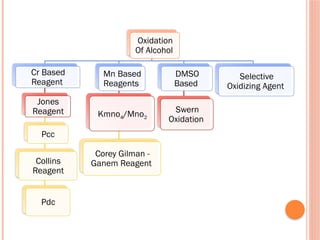
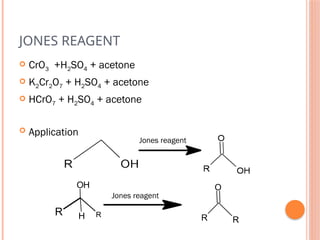
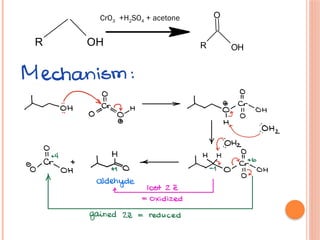
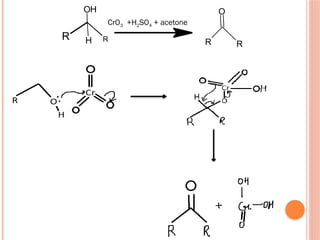
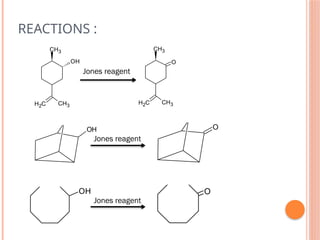
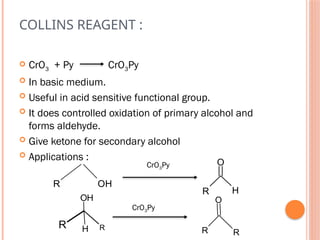
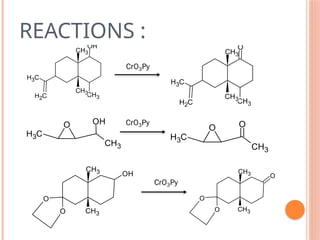
This PowerPoint presentation titled "Chromium (Cr) Based Oxidizing Reagents" offers an in-depth exploration of the role of chromium compounds as powerful oxidizing agents in chemical reactions. It covers the fundamental principles, oxidation mechanisms, and redox behavior of chromium in different oxidation states, particularly Cr(VI). The presentation discusses widely used reagents such as PCC (Pyridinium chlorochromate), PDC (Pyridinium dichromate), Jones reagent (Chromic acid), and Collins reagent, highlighting their preparation, reaction conditions, selectivity, and safety considerations. Real-world laboratory applications, environmental impact, and green chemistry alternatives are also discussed. This content is useful for chemistry students, academic professionals, and researchers involved in synthetic organic or inorganic chemistry.

![OXIDATION OF ALCOHOL
C
H3 OH
H
H
C
H3 H
O
C
H3
OH
O
Controlled
oxidation
Increase in o.s
Increase in o.s
Removal of H
Removal of H
R
OH
H
R R
O
R
[O]
-2 +2 +3
+2
0
R OH
Uncontrolled
oxidation :
R
O
OH](https://image.slidesharecdn.com/chromiumcrbasedoxidizingreagents-250721062957-955a63cf/85/Chromium-Cr-based-oxidizing-reagents-pptx-3-320.jpg)

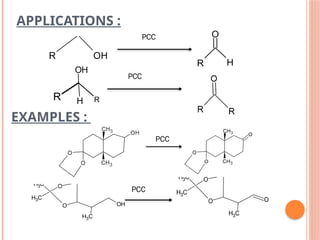
![PCC [ PYRIDINIUM CHLORO
CHROMATE ]
CrO3 + Py + HCl
Not moisture sensitive.
Excess reagent not required.
It is slight acidic but buffered by NaOAc.
Cr
O
O
Cl
O
–
N
+
H
PCC](https://image.slidesharecdn.com/chromiumcrbasedoxidizingreagents-250721062957-955a63cf/85/Chromium-Cr-based-oxidizing-reagents-pptx-12-320.jpg)
![BABBLER OXIDATION:
CH3
O
C
H3 OH
PCC can also oxidize tertiary alcohol
through [3,3] sigma tropic
rearrangement .](https://image.slidesharecdn.com/chromiumcrbasedoxidizingreagents-250721062957-955a63cf/85/Chromium-Cr-based-oxidizing-reagents-pptx-14-320.jpg)