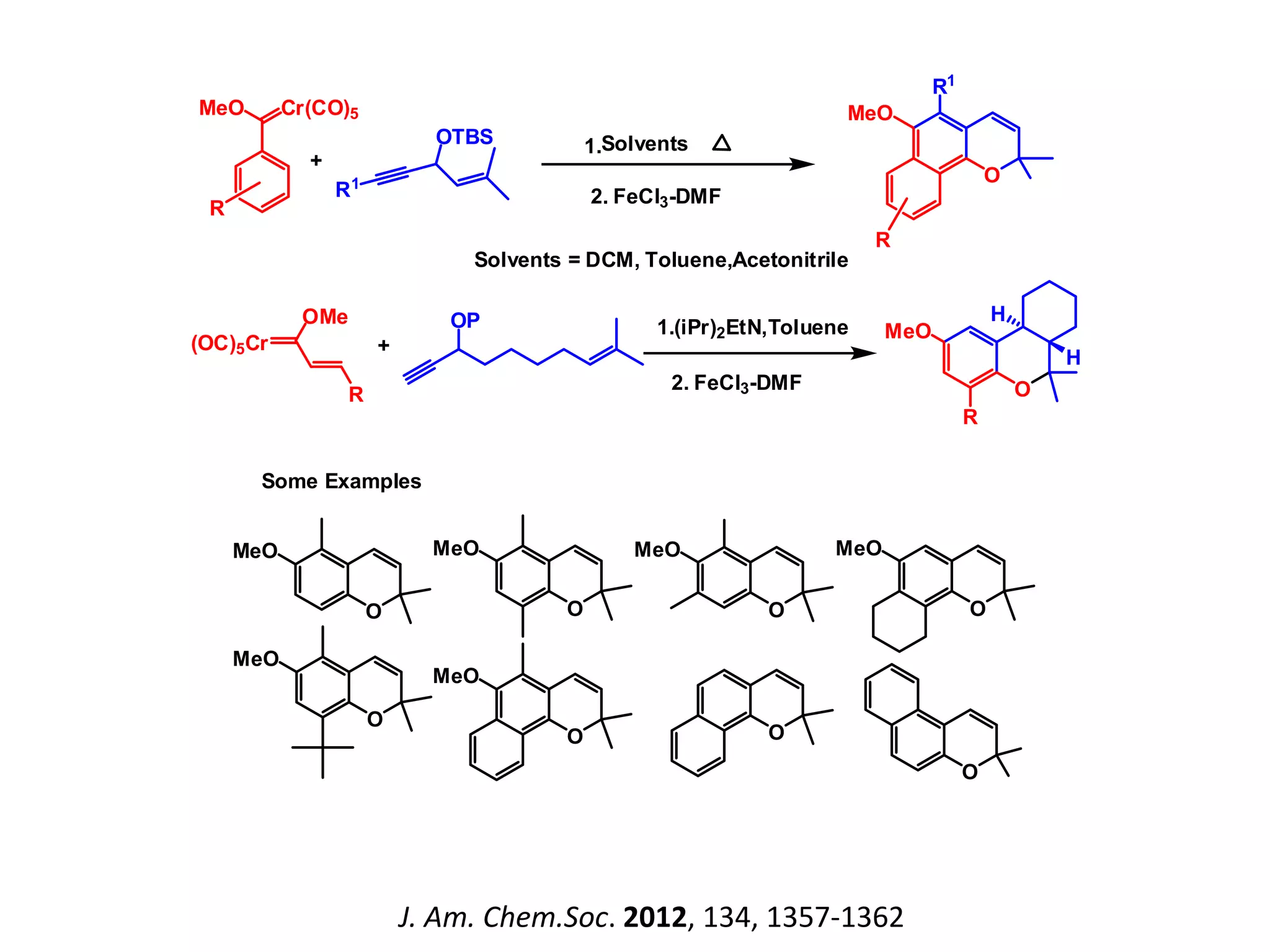
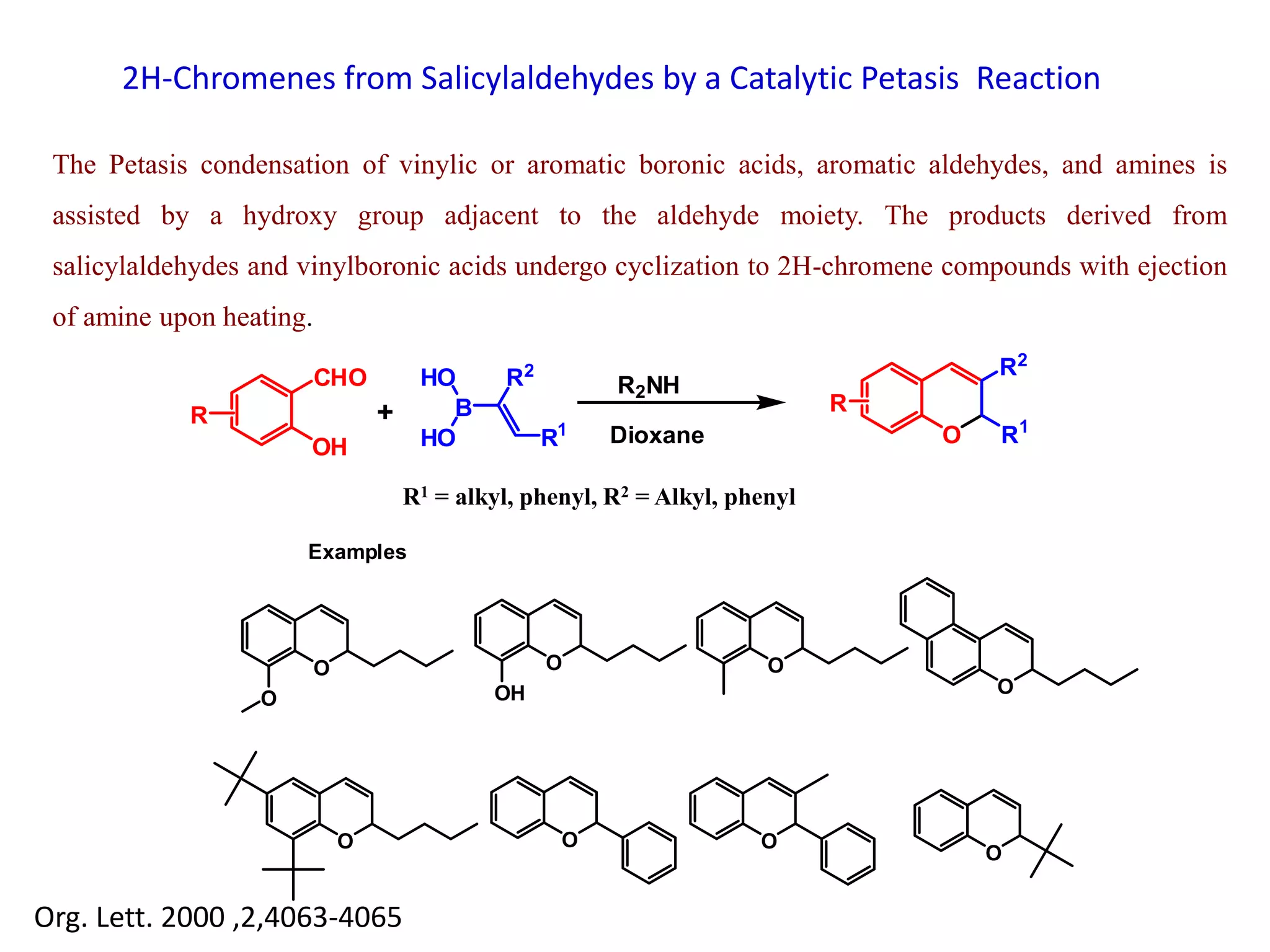
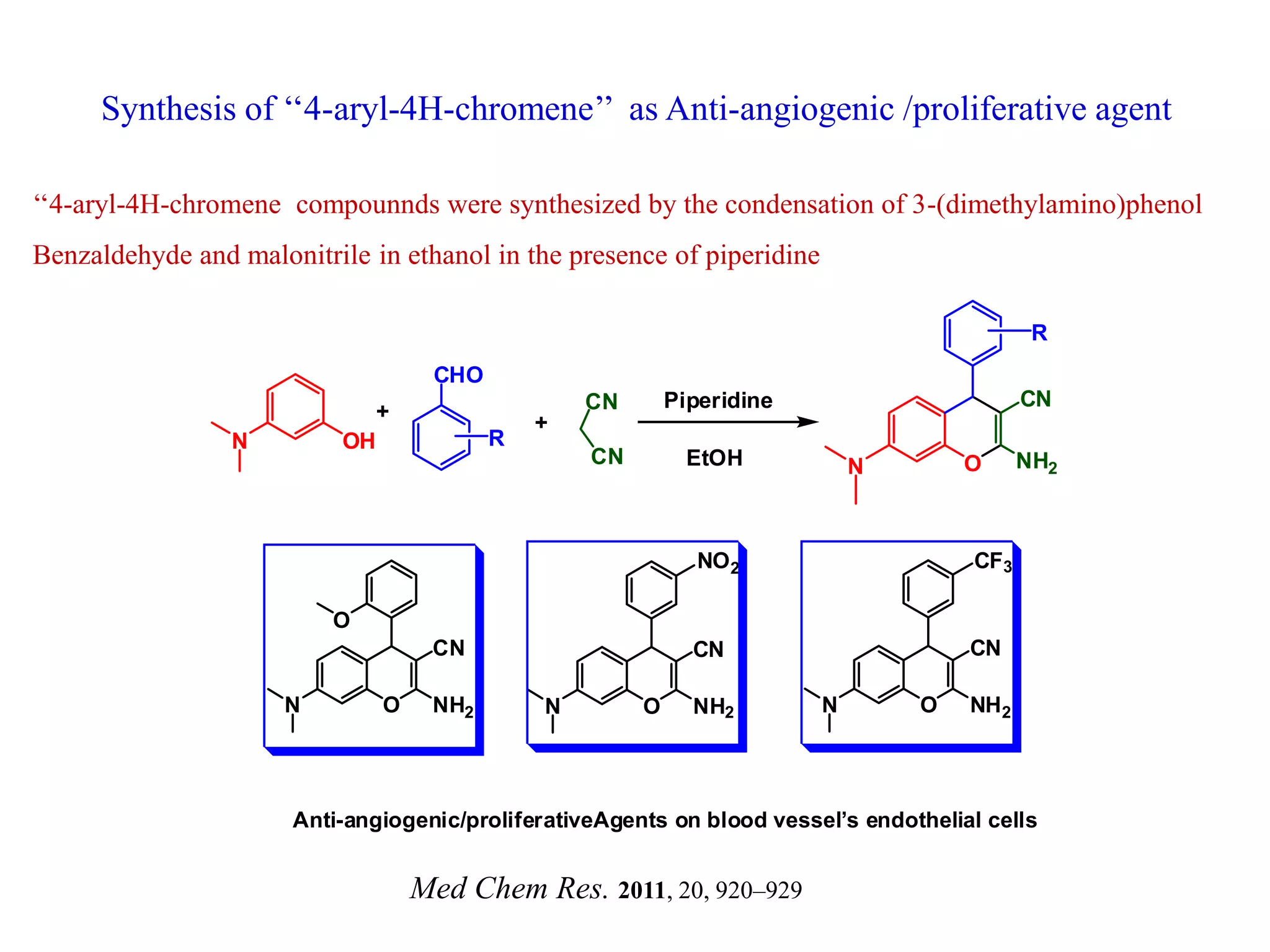
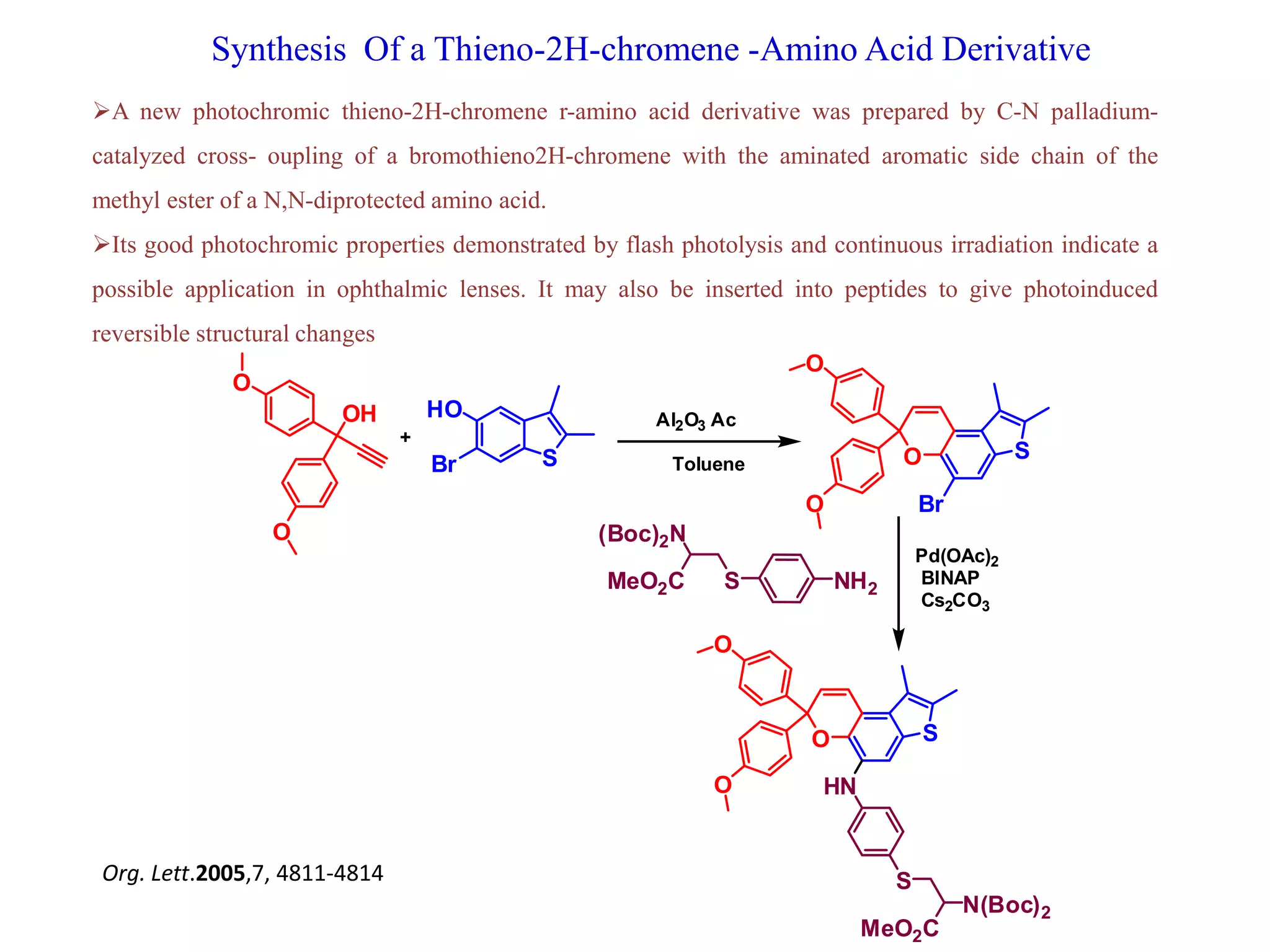
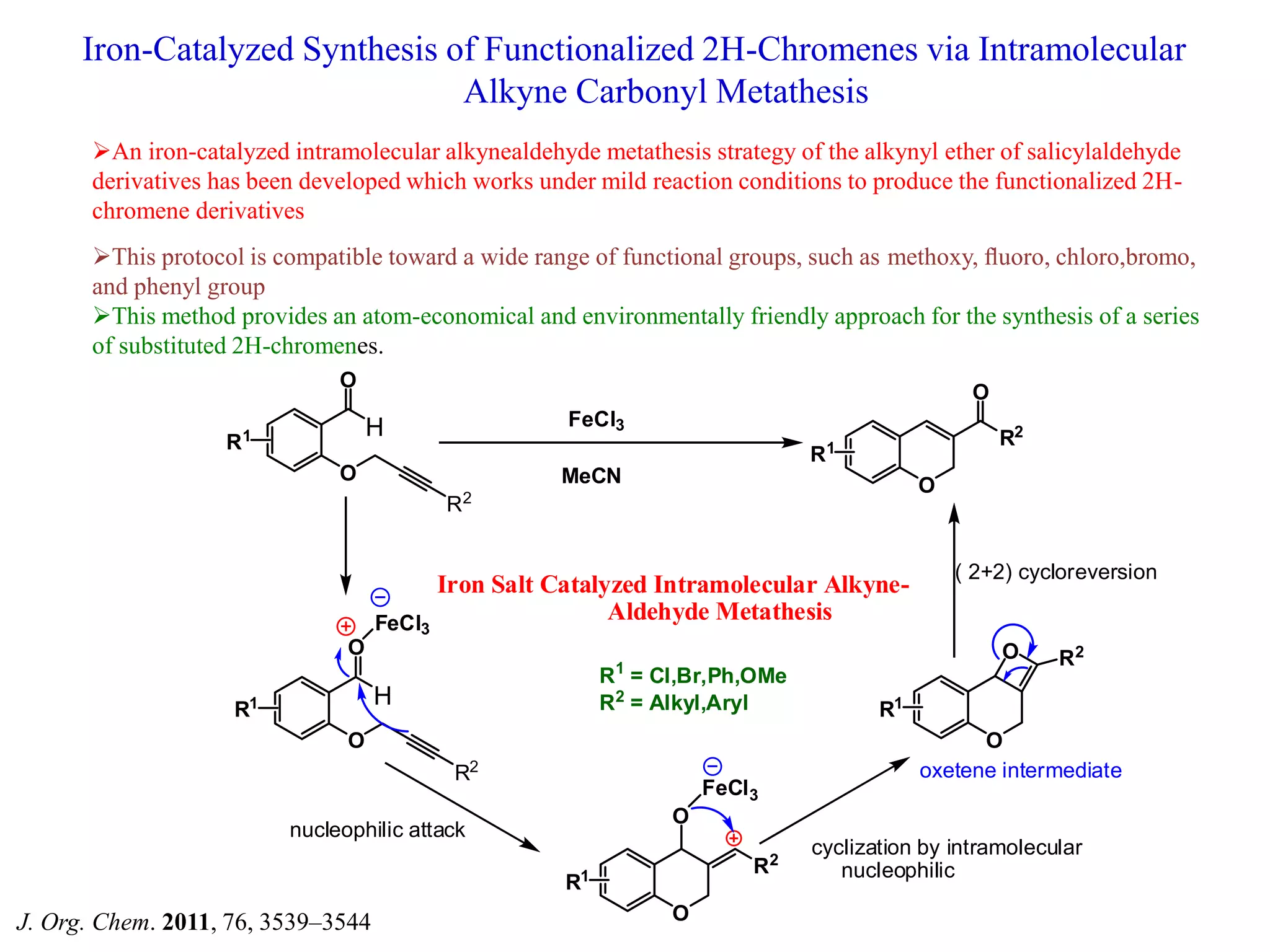
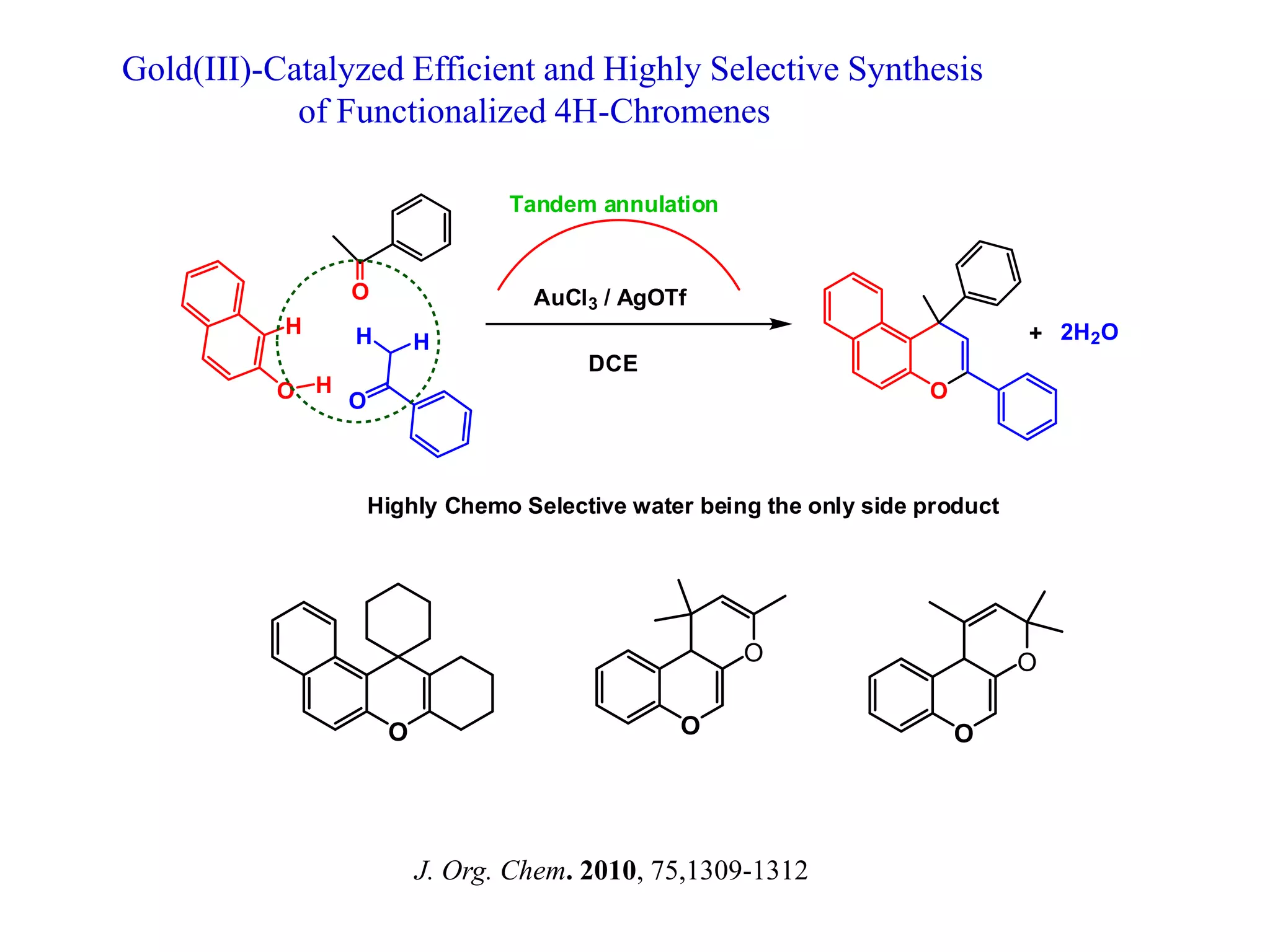
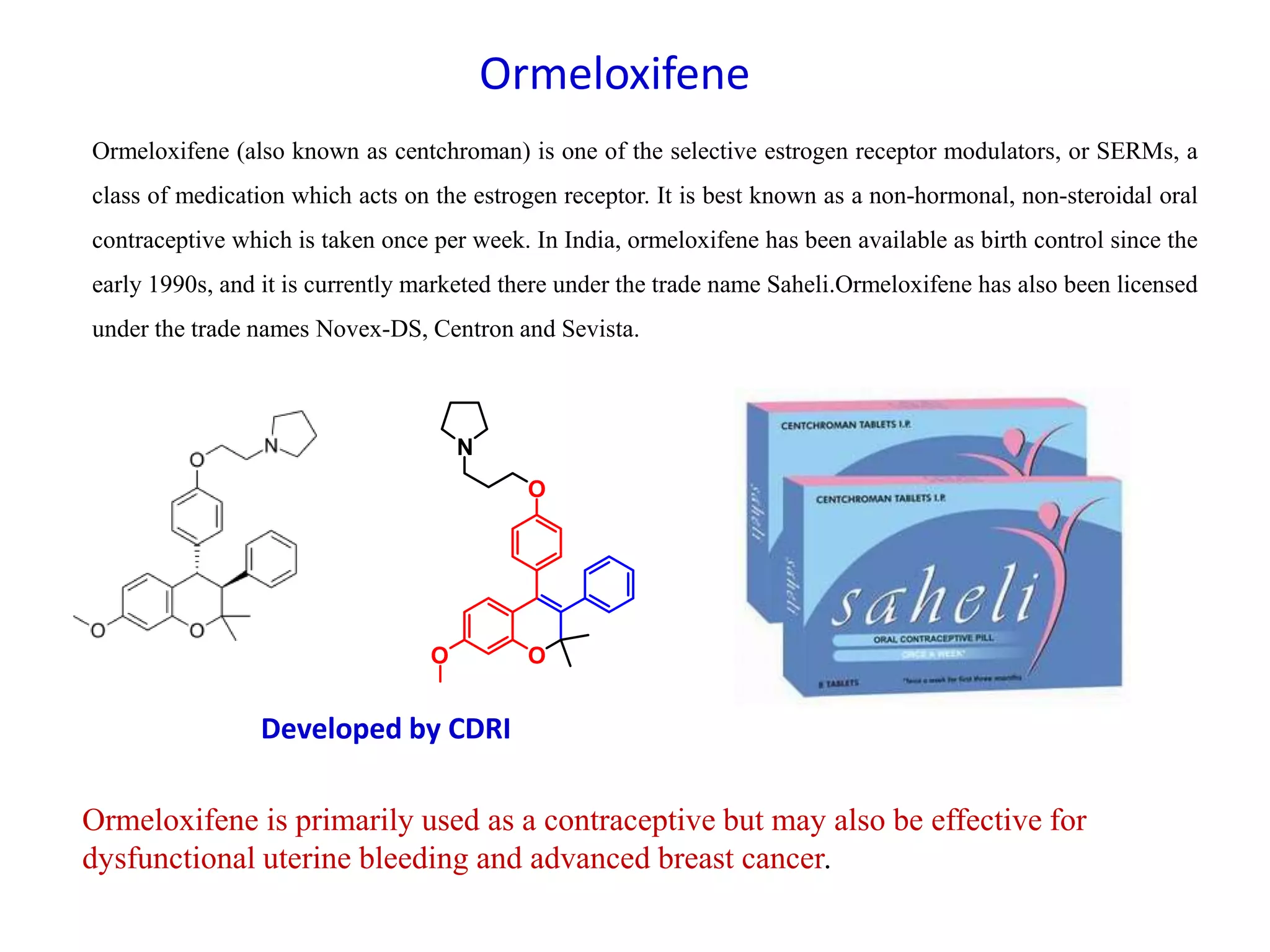
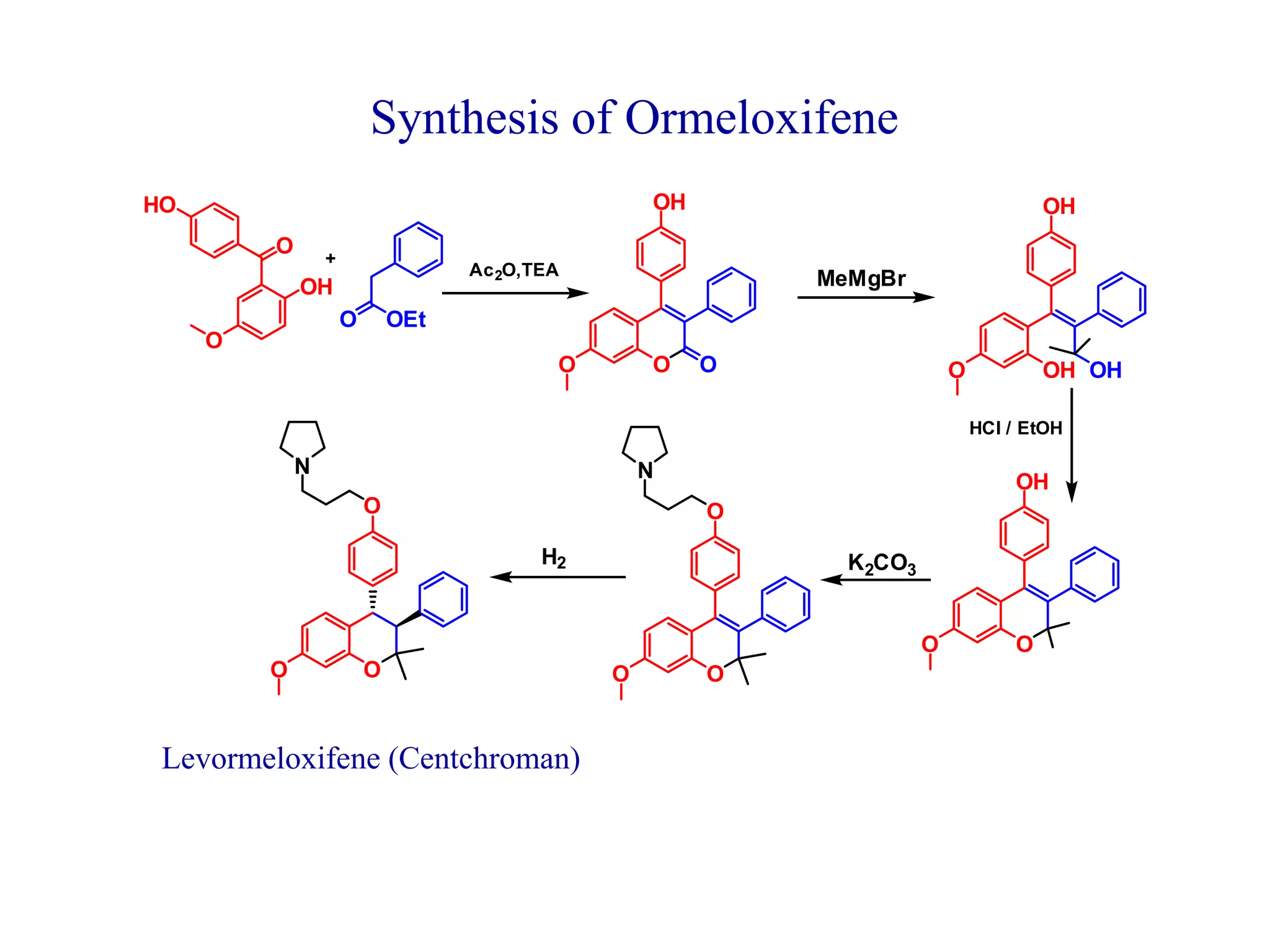
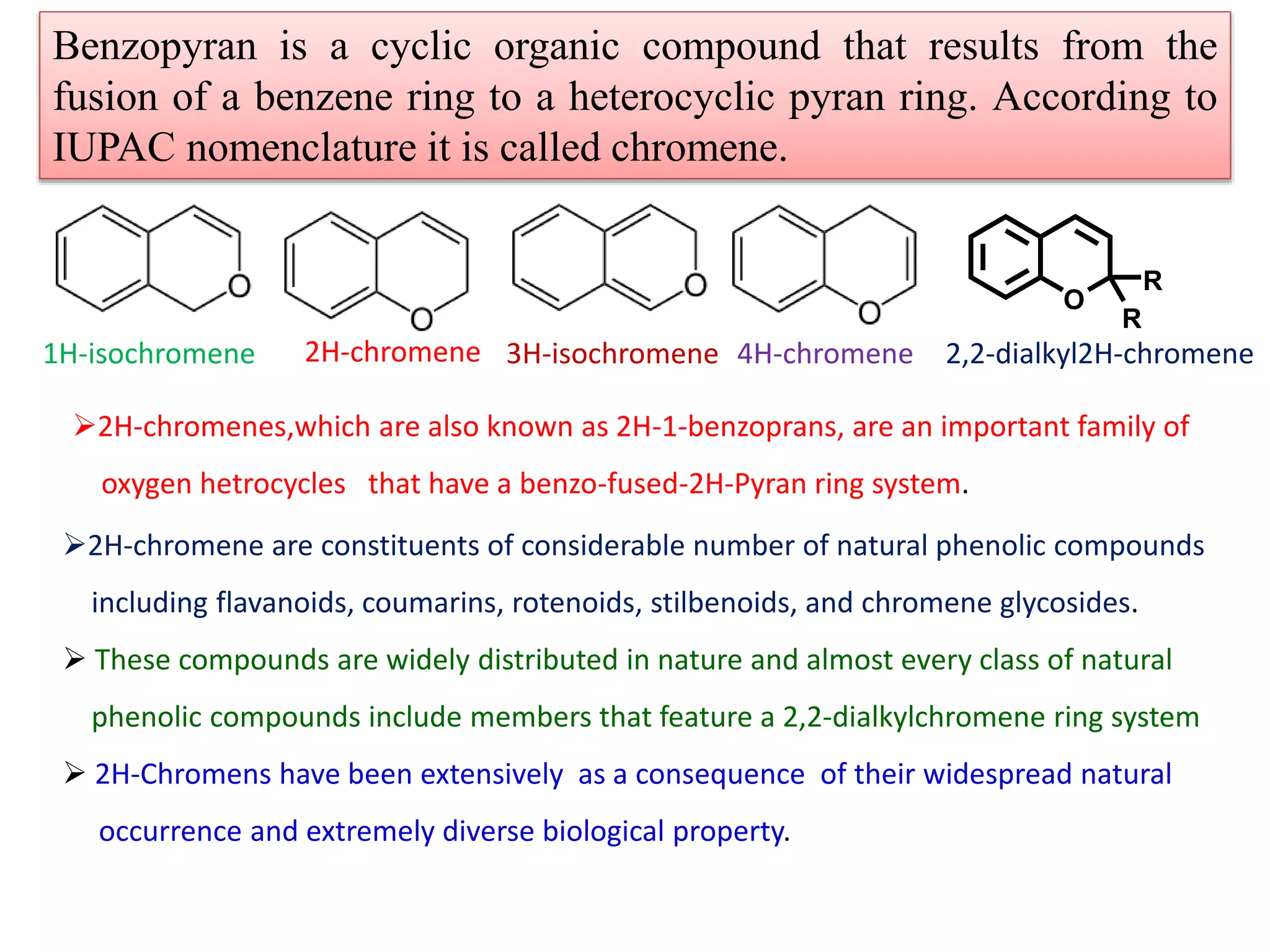
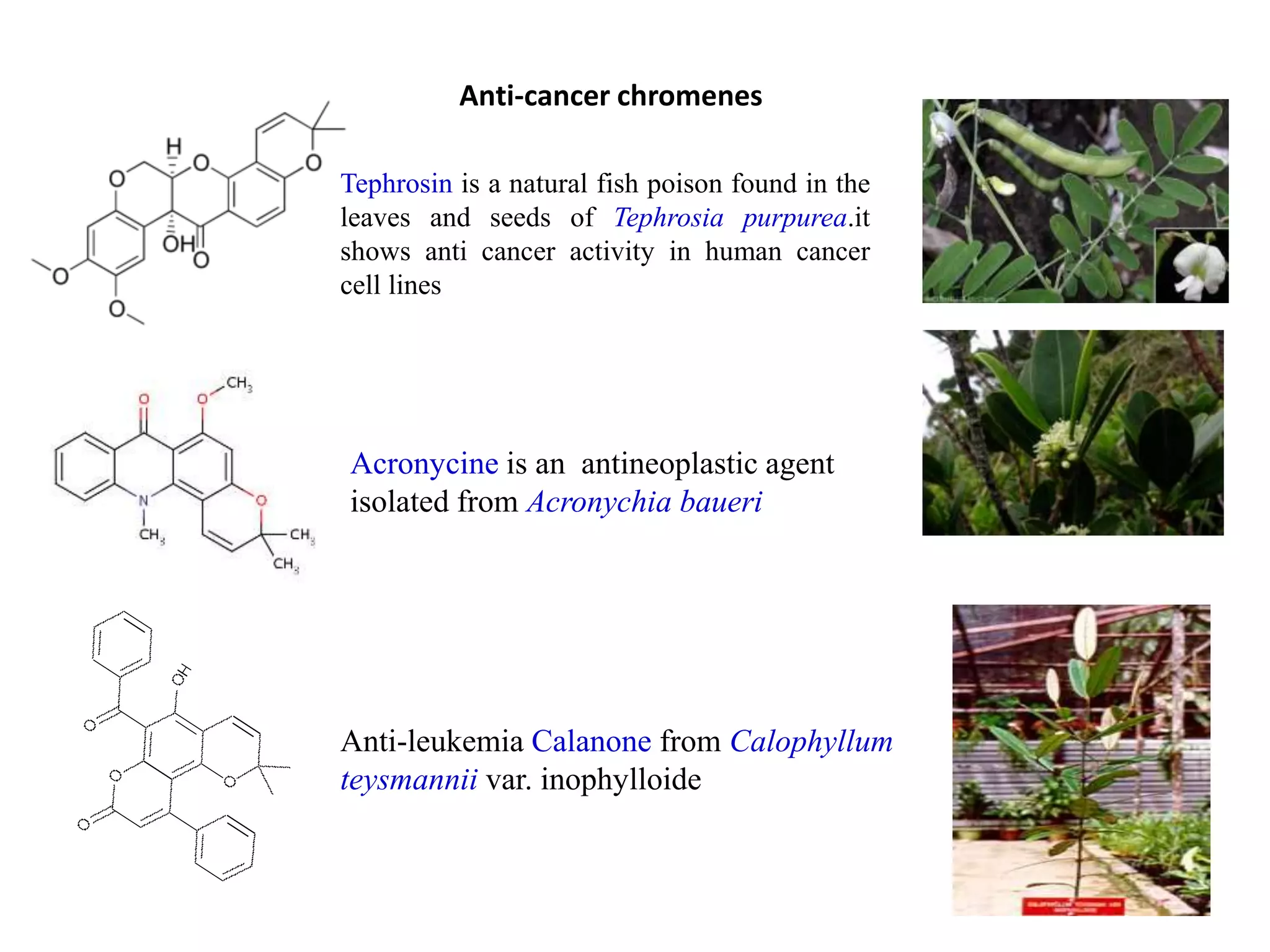
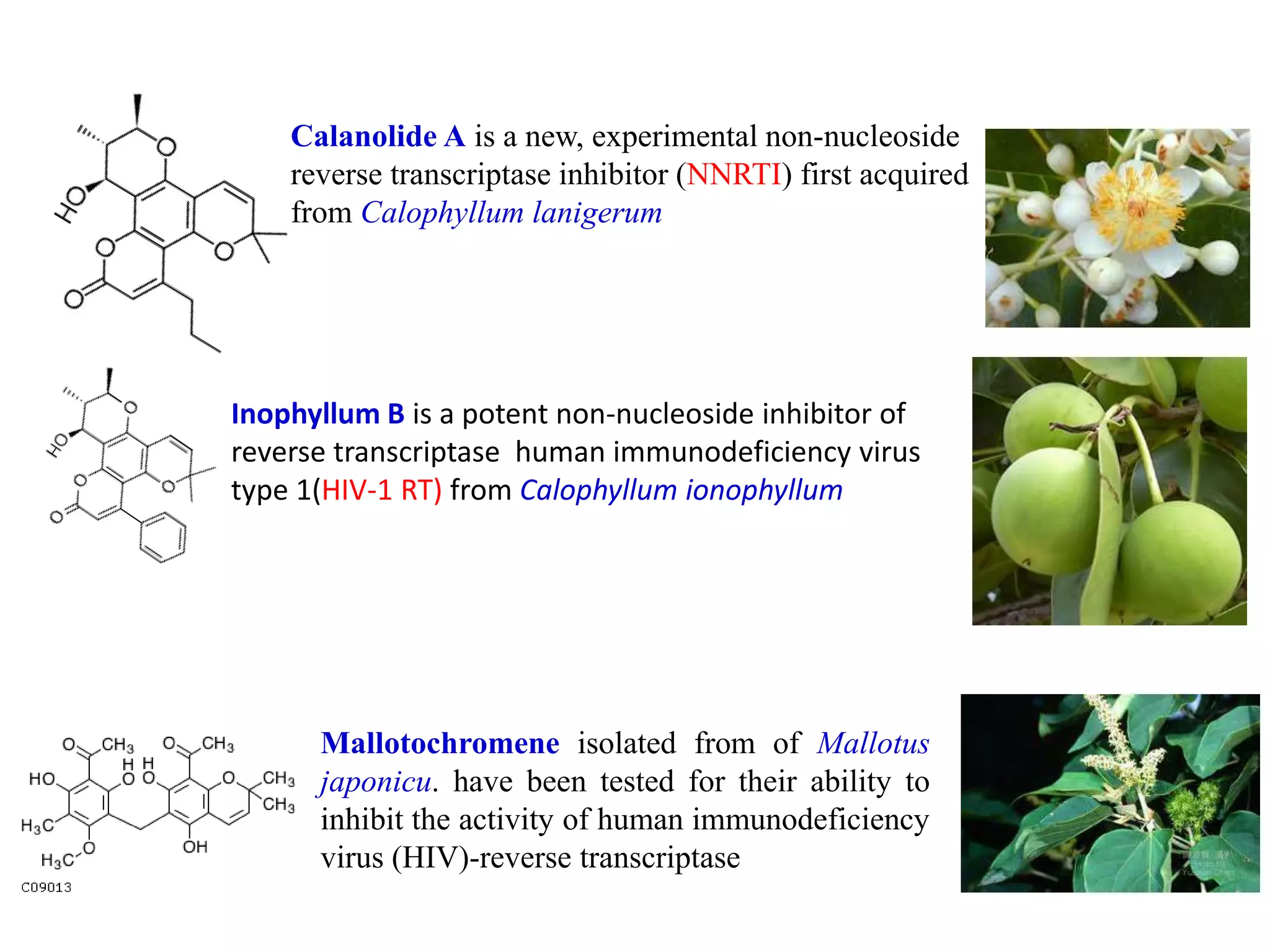
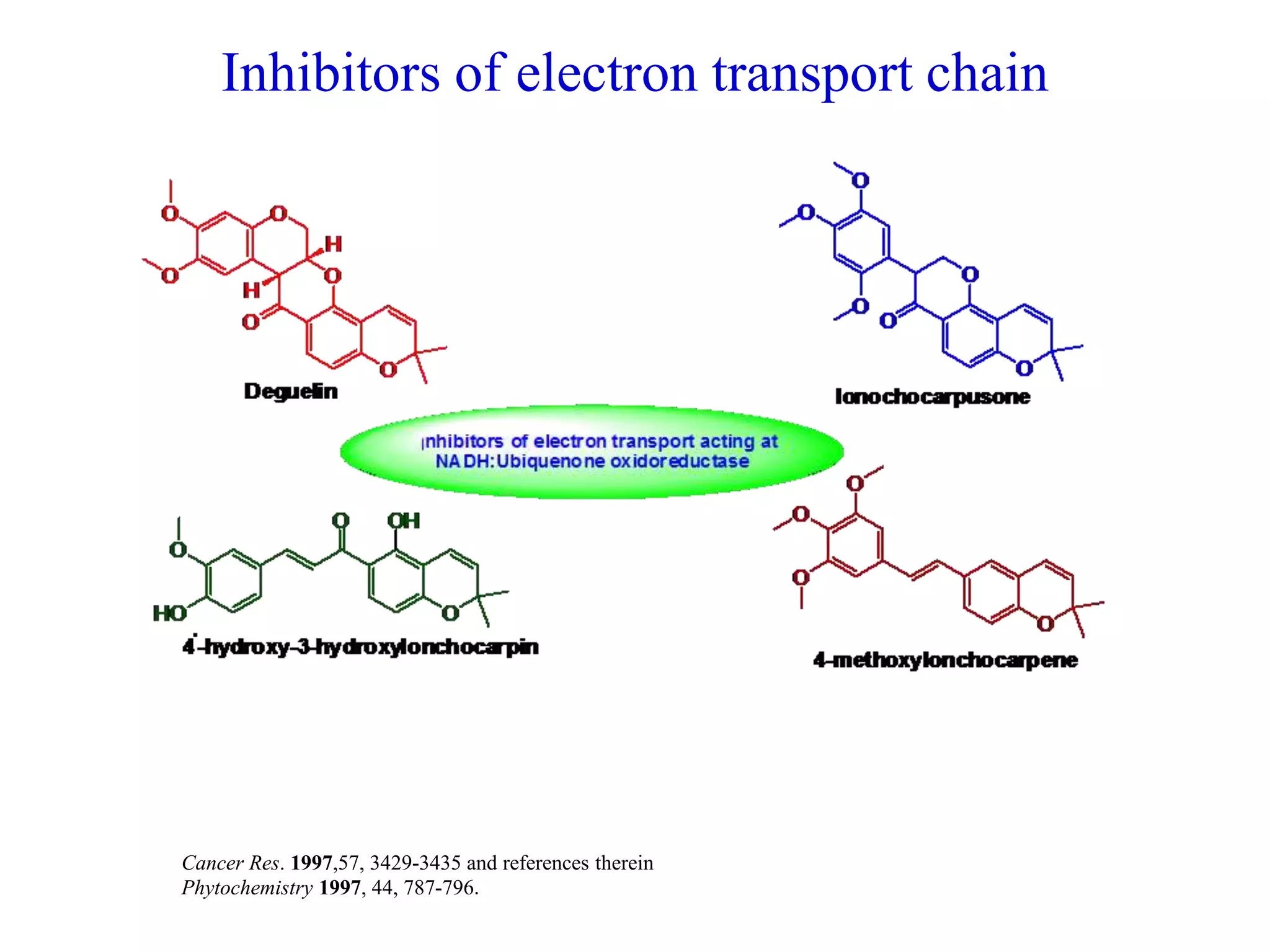
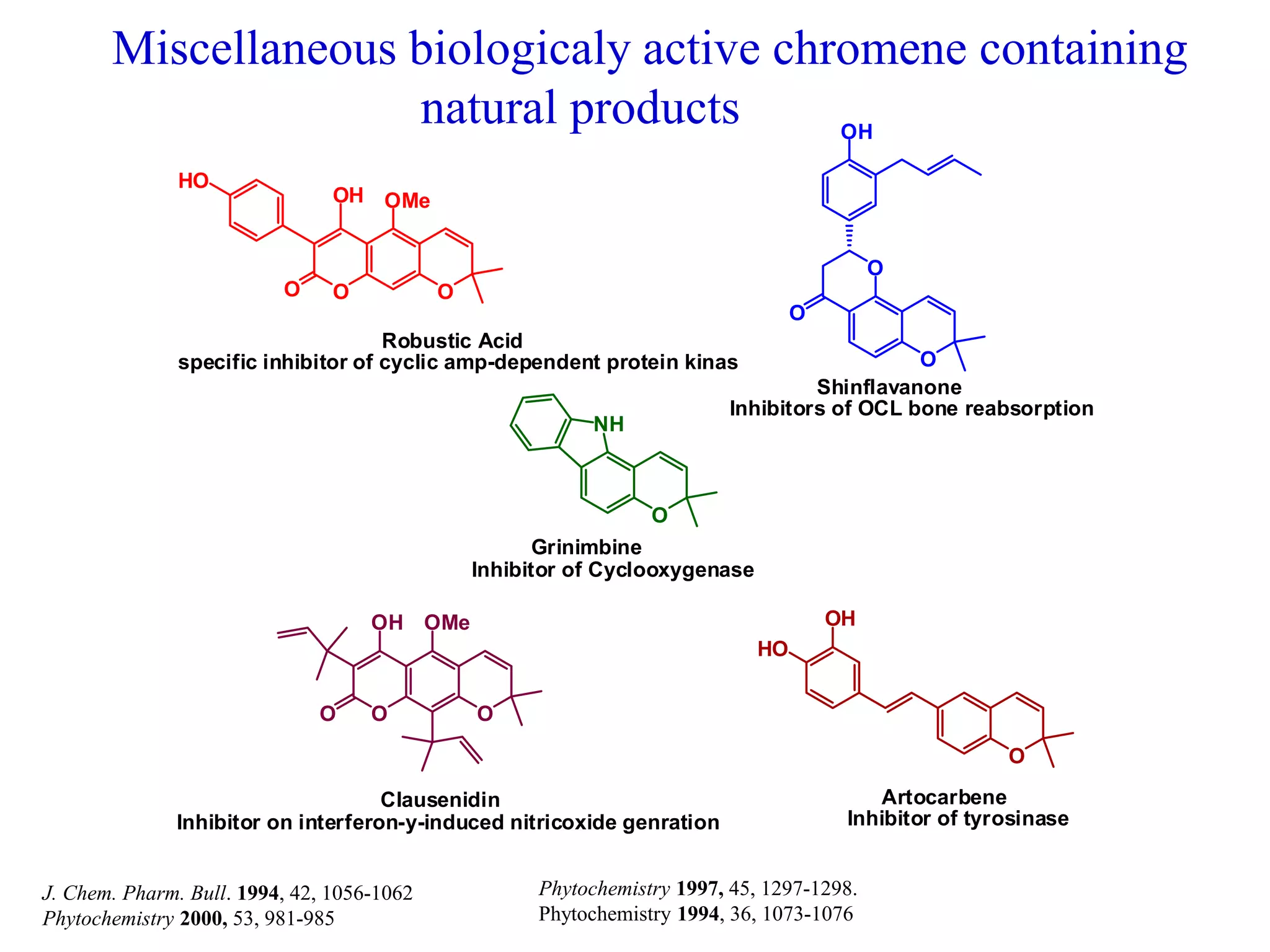
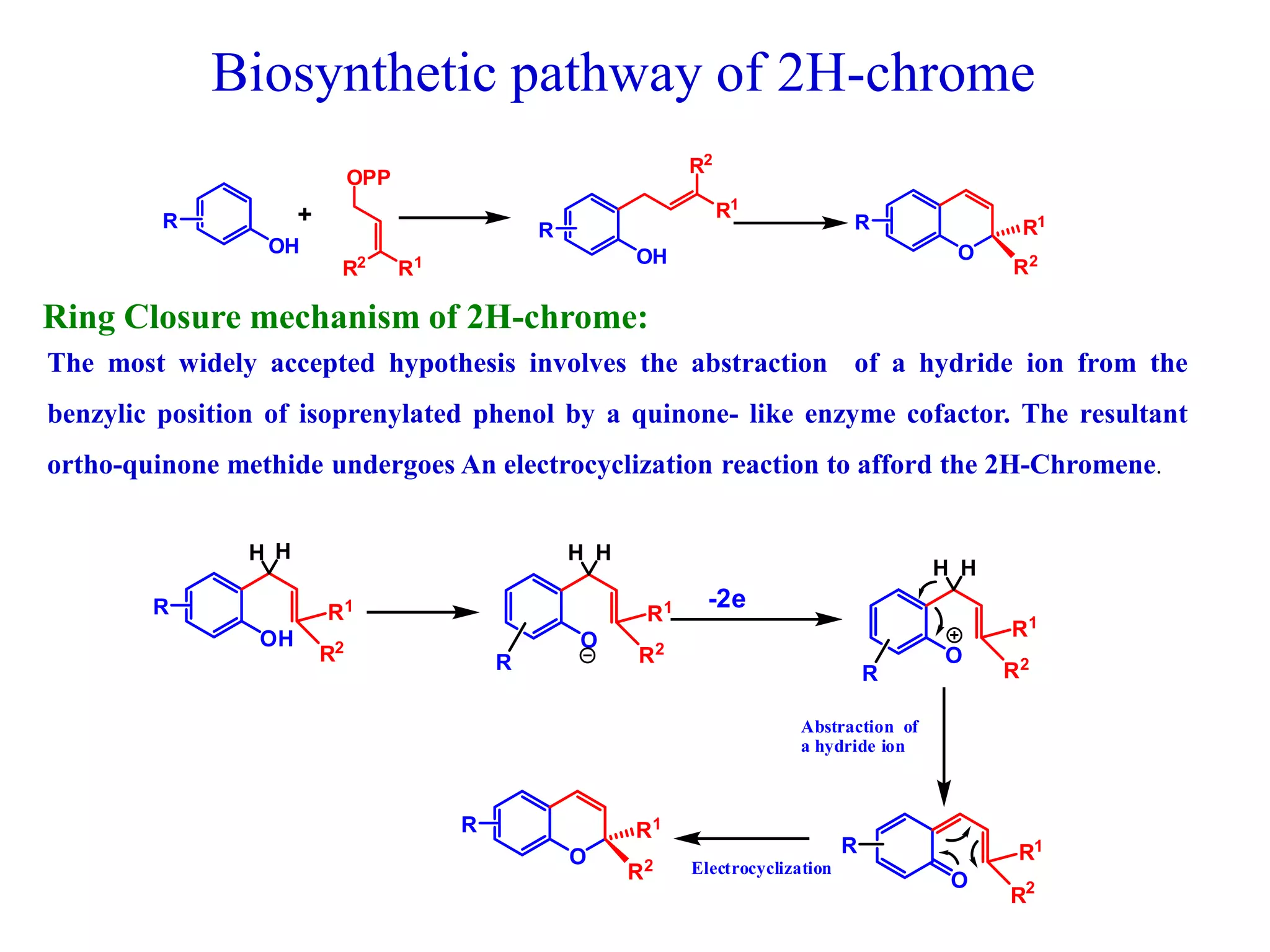
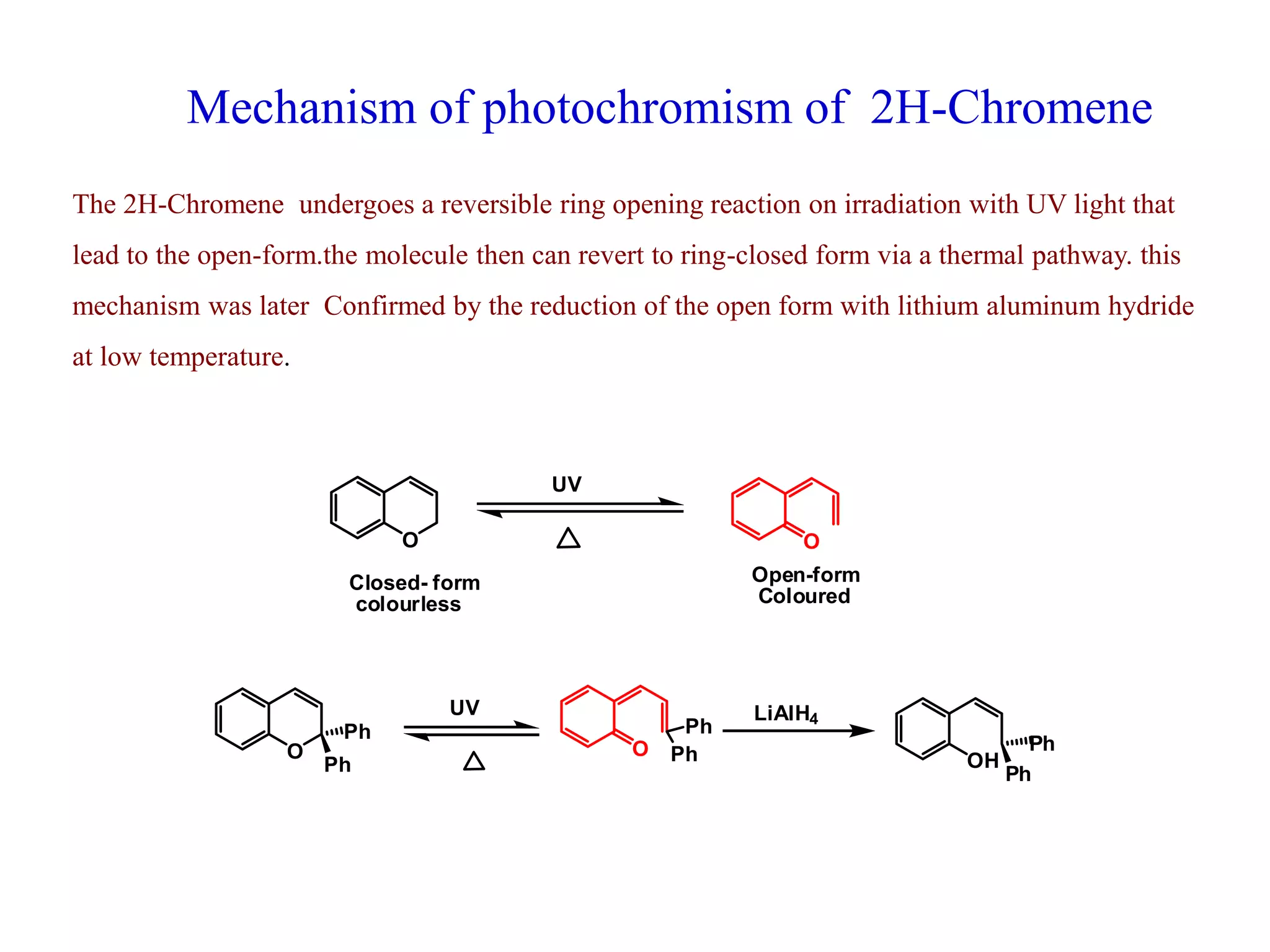
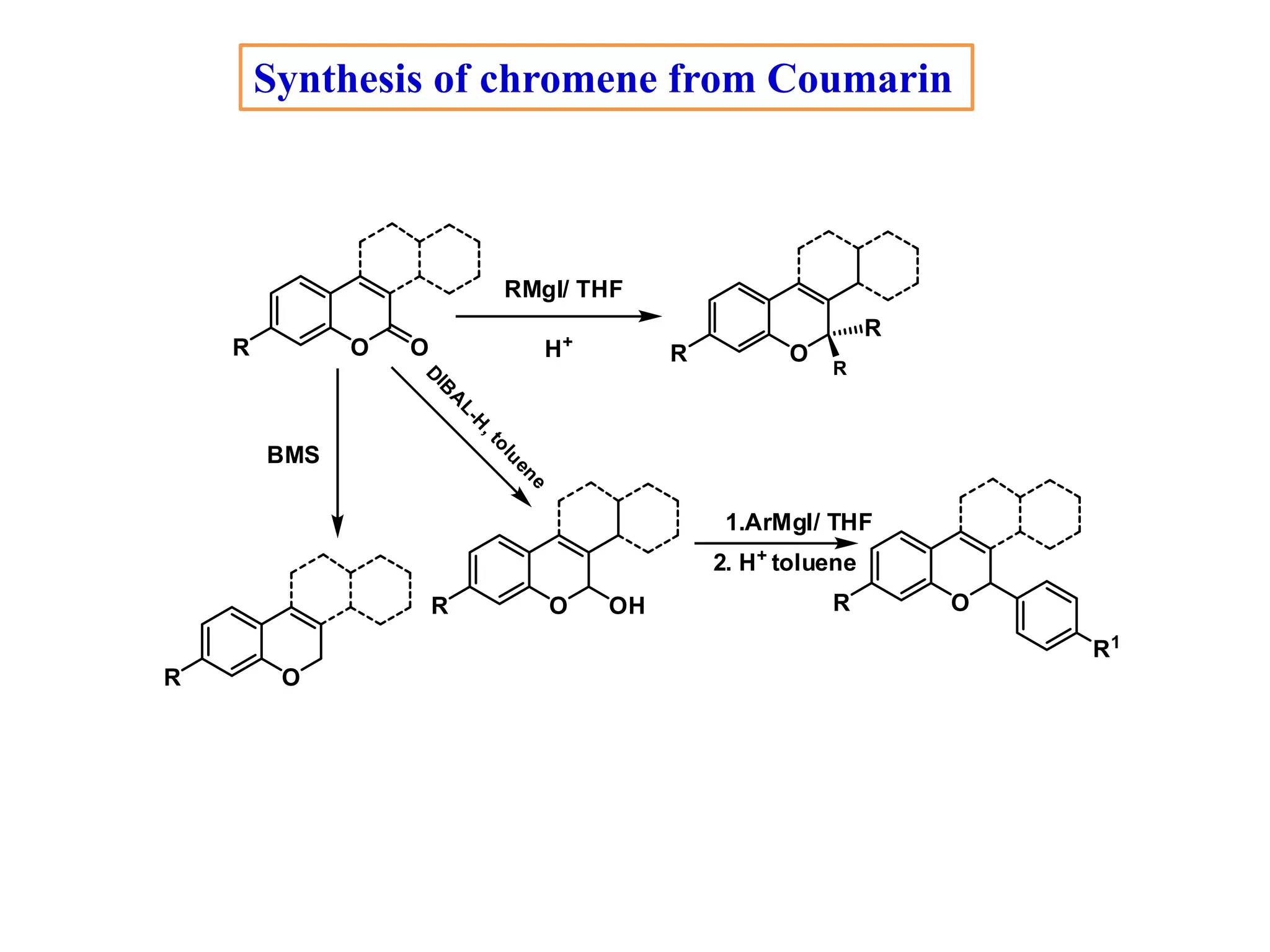
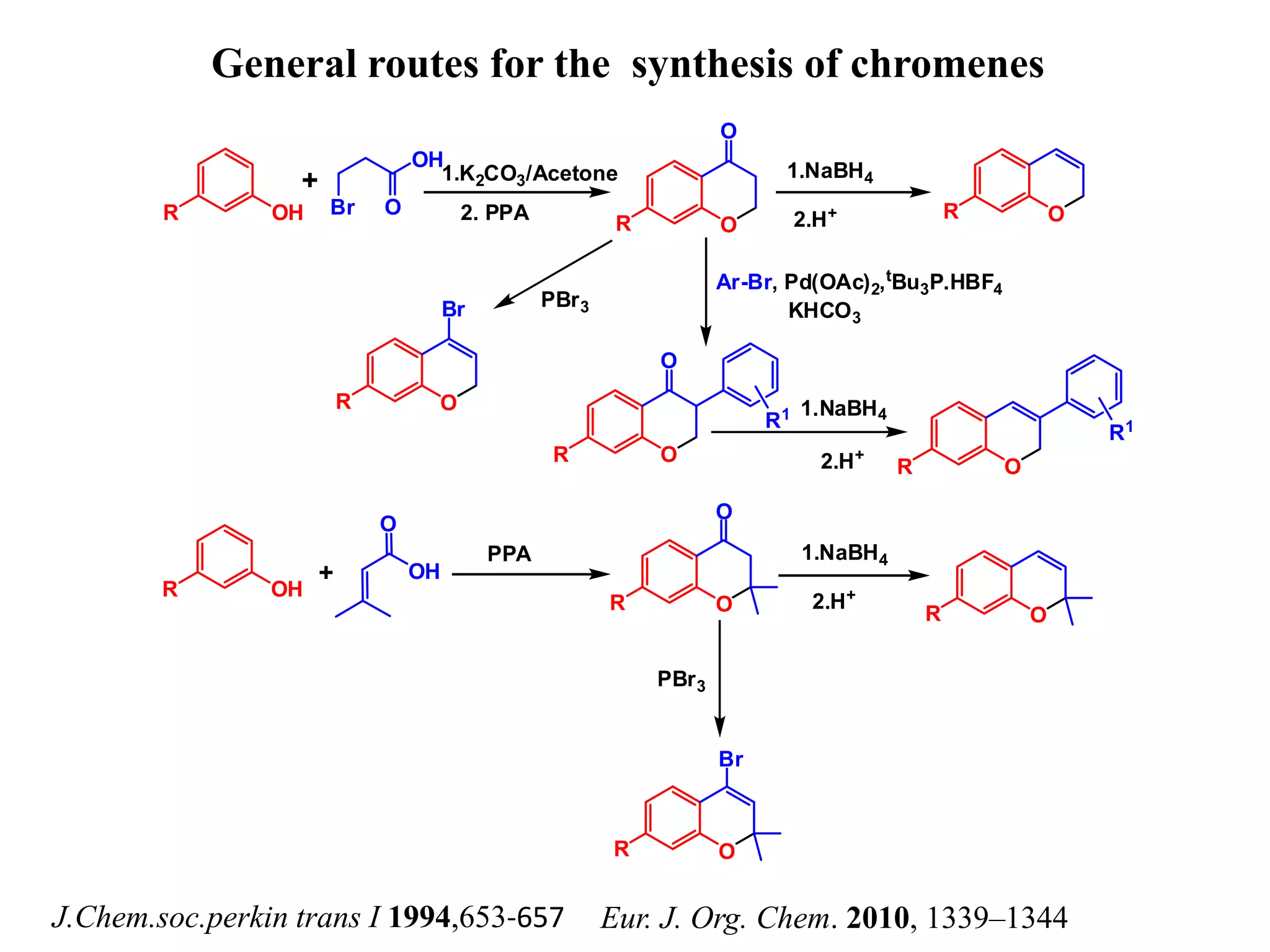
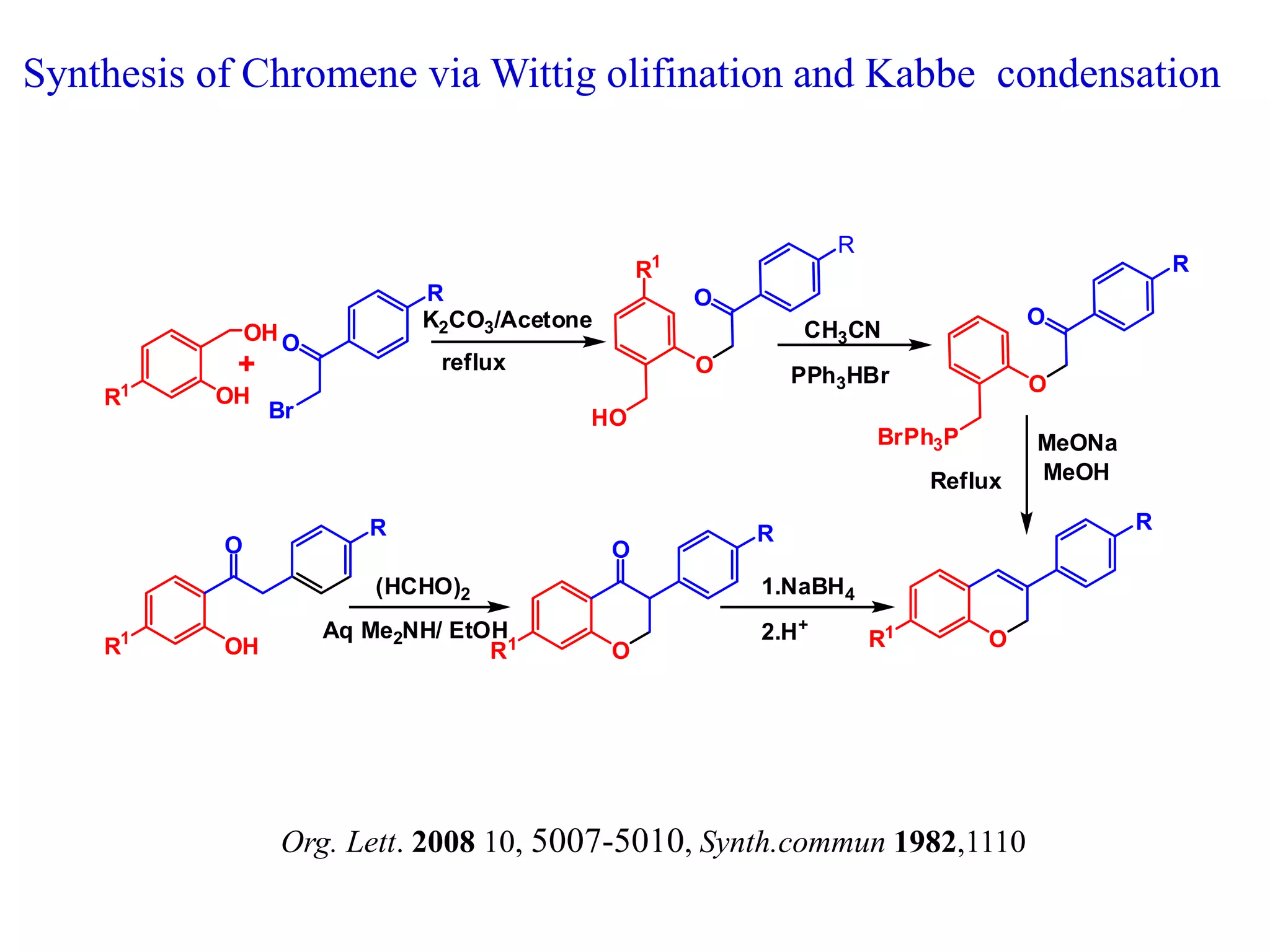
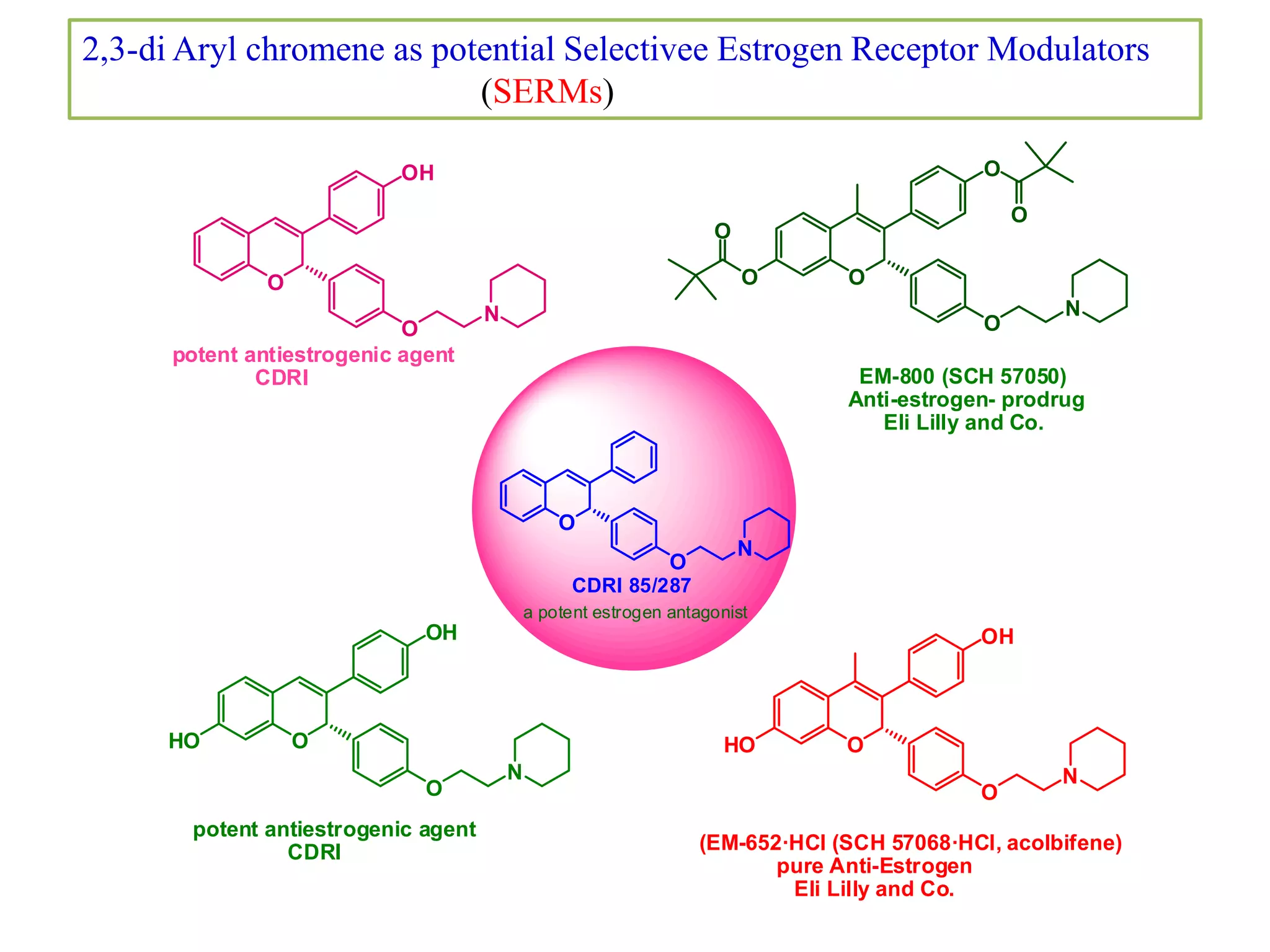
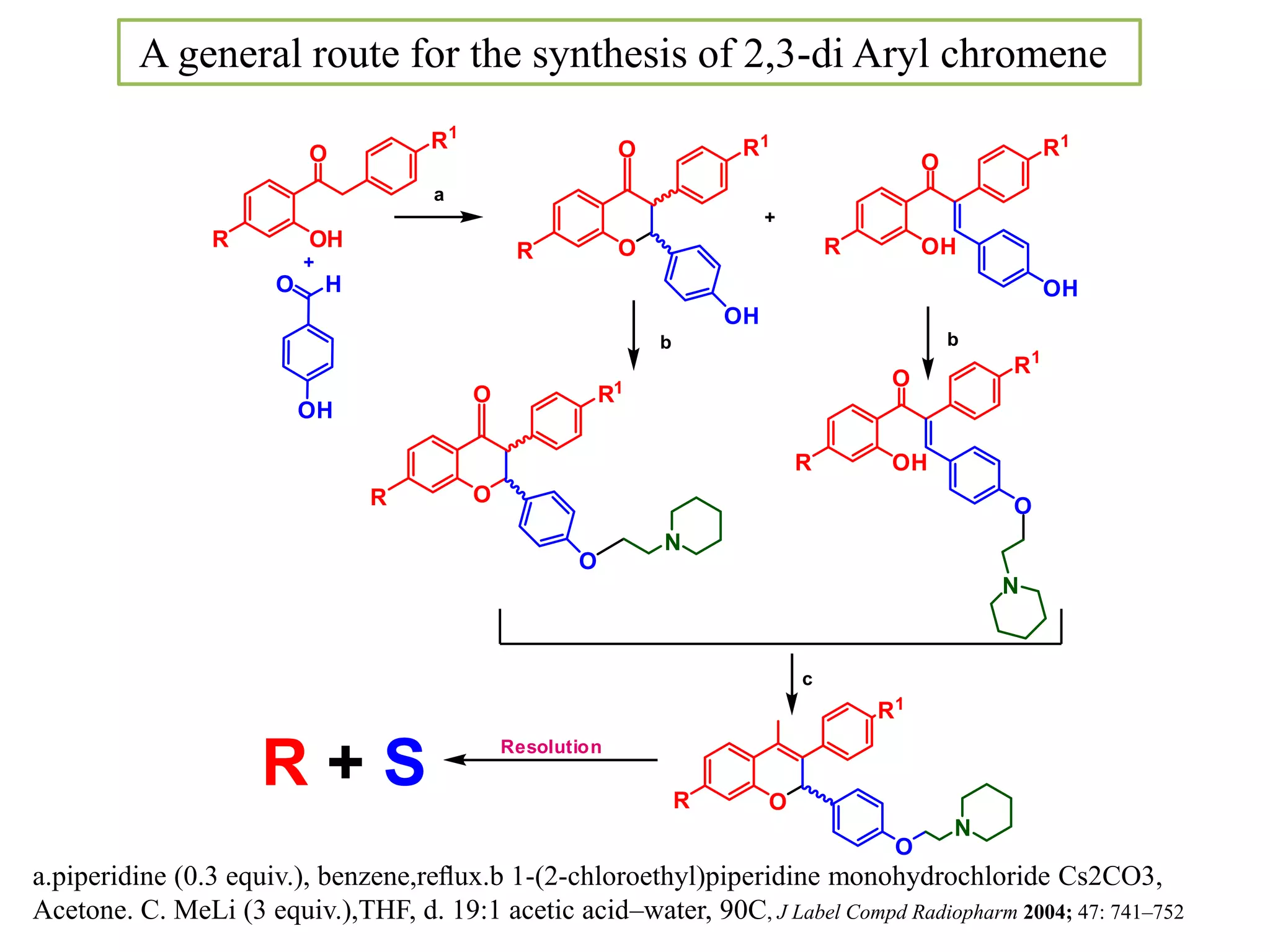
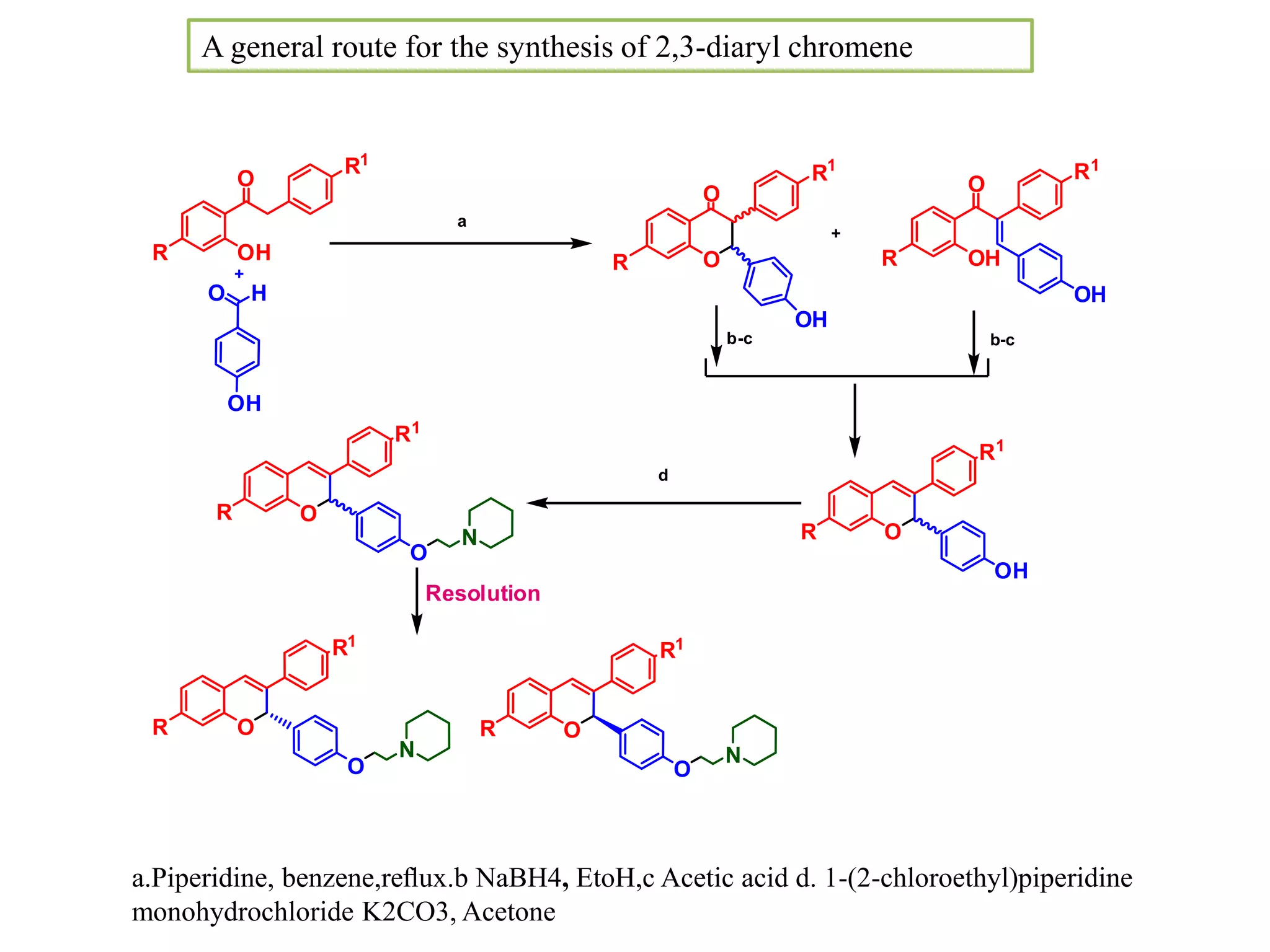
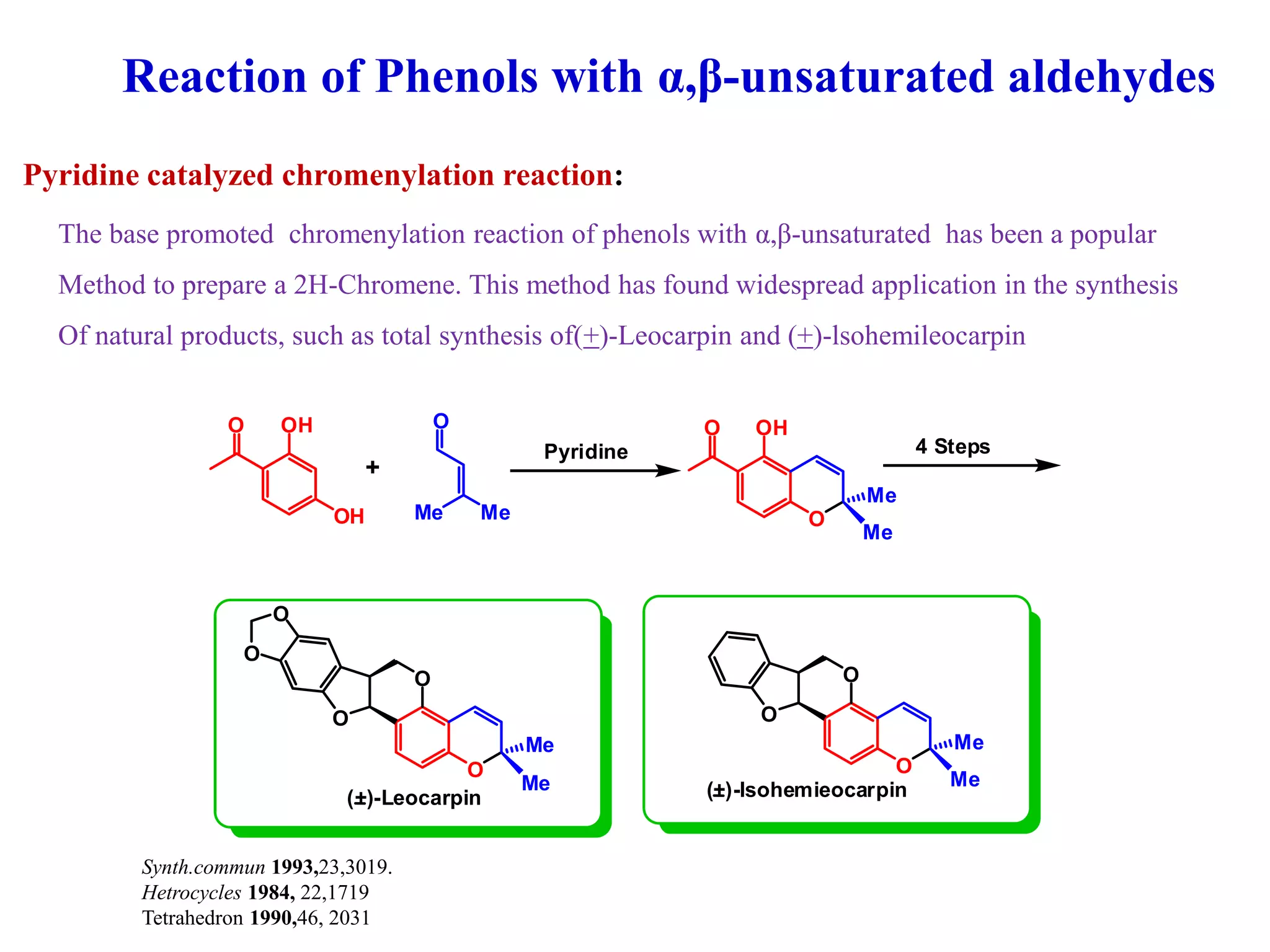
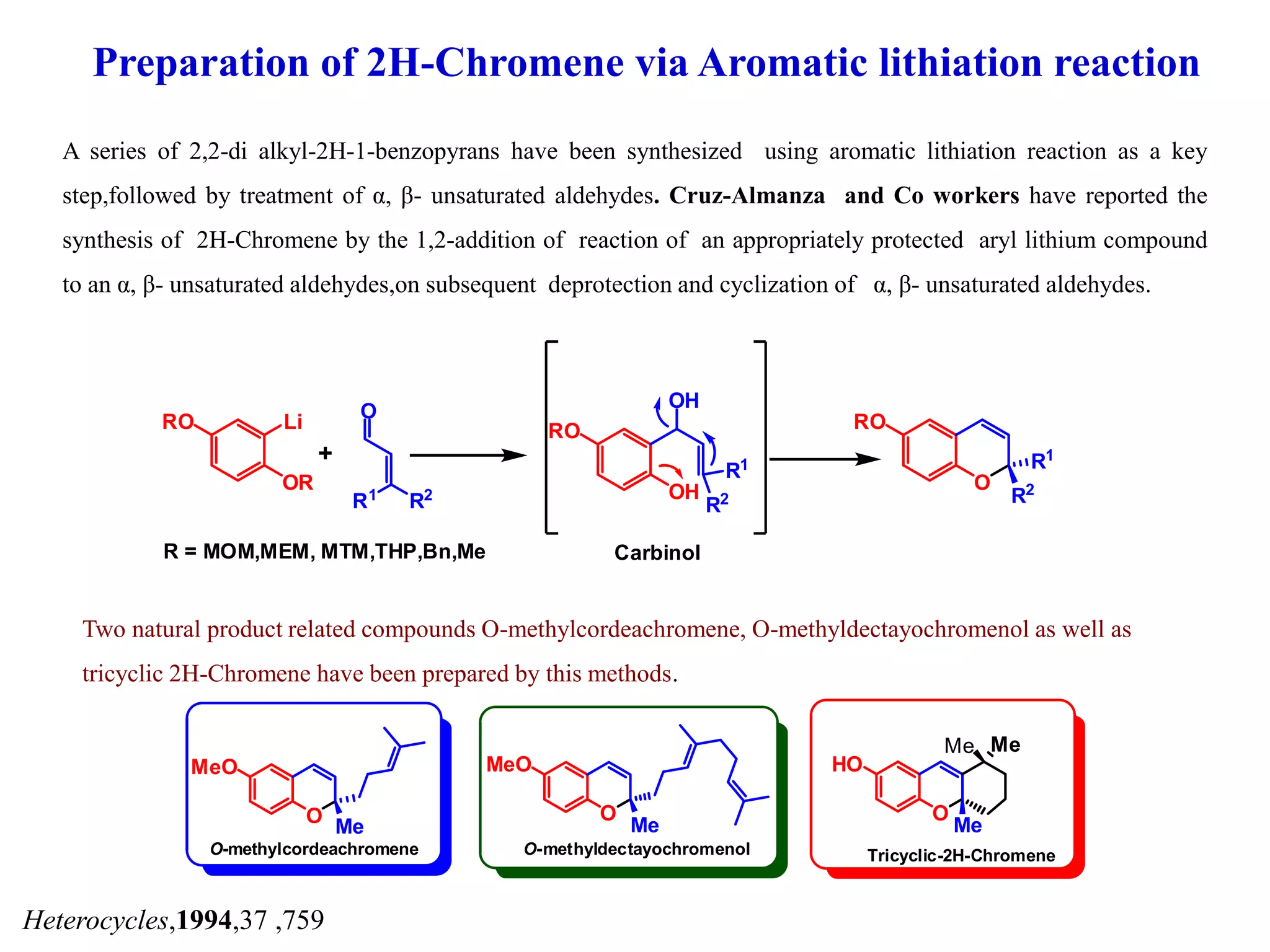
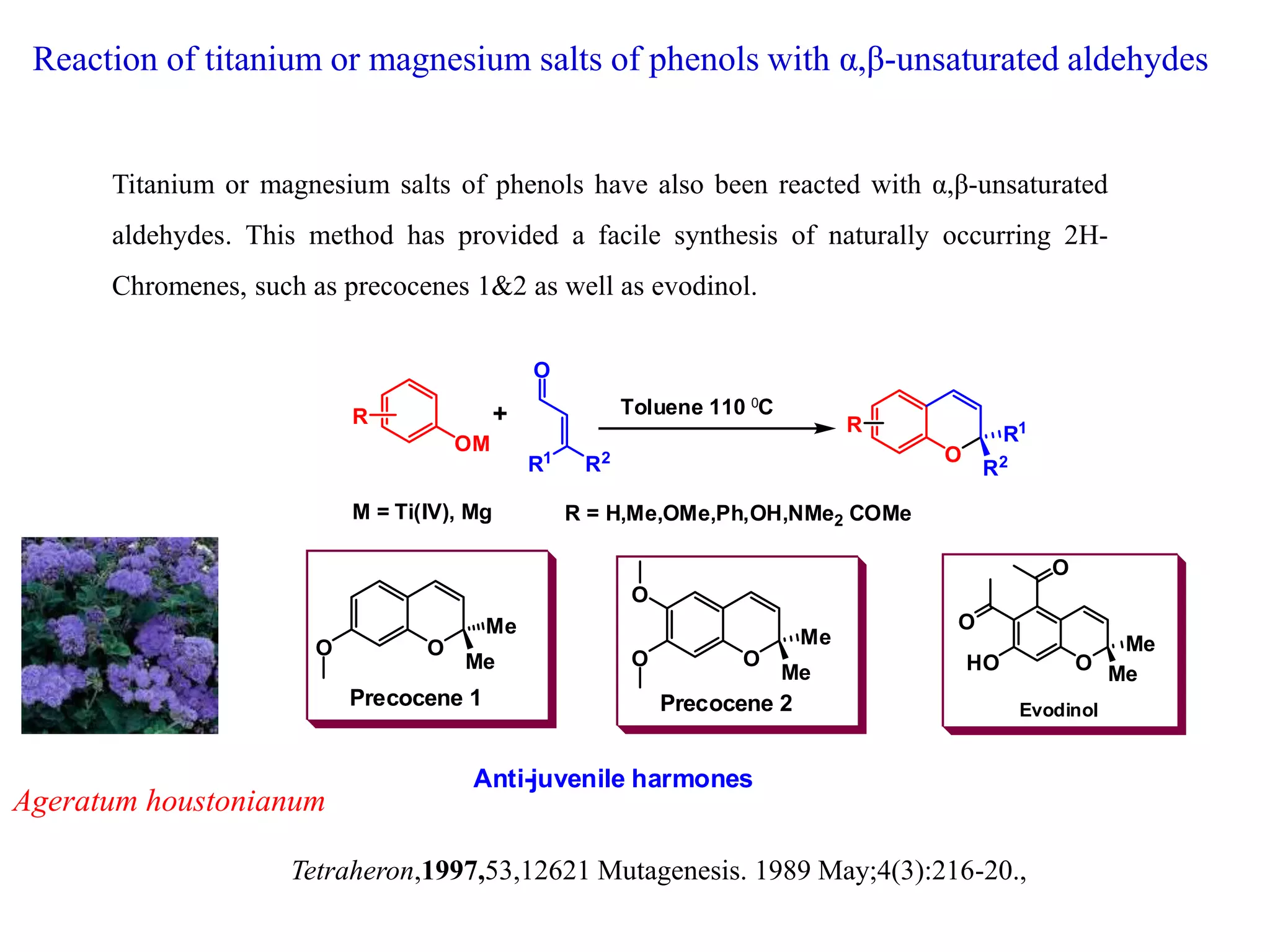
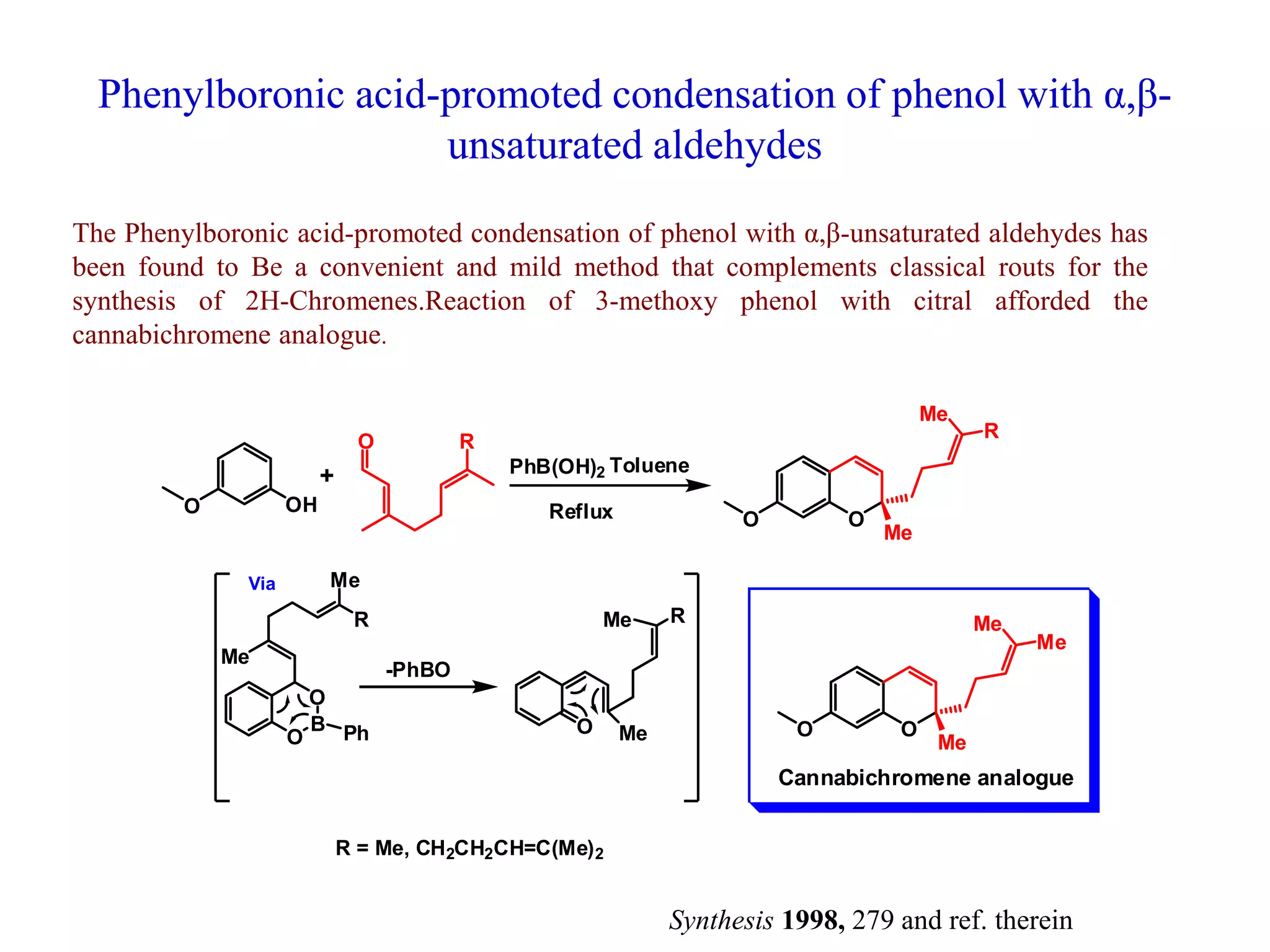
The document discusses the biological significance and synthesis of chromenes, a class of cyclic organic compounds with various natural phenolic derivatives. It highlights the diverse biological properties of 2H-chromenes, including their roles in cancer treatment and anti-inflammatory effects, and outlines various synthetic routes for their preparation. Additionally, the document covers specific examples of chromene compounds and their pharmacological applications.
![Preparation of 2H-Chromene via rearrangement of
propargyl Ethers
R
OH
R1
R2
R3
X
R
O
R1
R2
R3
O
R2
R3
R1
R
K2CO3 / Acetone
reflux
PhNMe2
reflux
+
R
O
R1
R2
R3
O
R1
C R2
R3
R
O
R1
C R2
R3
R
H O
R2
R3
R1
R
Via
X = Cl,Br, R = H OMe, Me,Cl,CN,NO2
A particularly useful synthesis of 2H-Chromenes involves the thermal rearrangement of
propargyl Ethers in a solvents of high boiling point. the reaction is proposed to proceed via
claisen- like [3,3]-sigmatropic rearrangement,followed by a[1,5] sigmatropic shift.An
electrocyclization reaction then complete the process
Tetrahedron.lett,2001,42,1091](https://image.slidesharecdn.com/chromene-200412154041/75/Chromene-Synthesis-and-Medicinal-properties-23-2048.jpg)
![Ring-Closing Olefin Metathesis
Ring-Closing Olefin Metathesis has been developed into a practical and highly efficient procedure for
a diverse array of 2H-Chromene derivatives . A series of substituted 2H-Chromenes were prepare in
high yields by the ring closing metathesis using a ruthenium carbene catalyst.
J. Org. Chem. 1998, 63, 864-866
R2
OH
O
R1
R3
R4
O
R1
R2
R4
R3
X
O
R2
R3
R4
R1
1.K2CO3 / Acetone
2. MTPPB,NaH
[Cl2(PCy3)2Ru=CHPh]
RCM
R1
/ R2
/ R3
/ R4
= H /Alkyl /Cl/ or diffrent sibstituens
+
Ru
Ph
PCy3
PCy3
Cl
Cl](https://image.slidesharecdn.com/chromene-200412154041/75/Chromene-Synthesis-and-Medicinal-properties-24-2048.jpg)