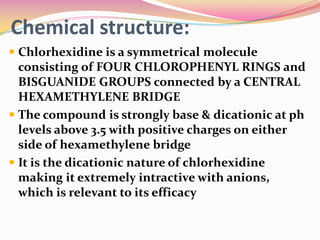
Chlorhexidine is a gold standard chemical plaque control agent with broad-spectrum bactericidal properties. It has a symmetrical molecule structure connected by a central bridge. Chlorhexidine exists in digluconate, acetate, and hydrochloride salt forms, with digluconate being most common. Its mechanism of action involves adsorption to bacterial cell walls, causing cell leakage and death at high concentrations. It has long-lasting substantivity due to its dicationic nature binding to teeth and bacteria. Chlorhexidine is used as an oral rinse, gel, or in other forms to reduce plaque and gingivitis as an adjunct to oral hygiene.



![Mechanism of action:
Antimicrobial activity:
CHX shows different effects at different concentrations
The agent is bacteriostatic, whereas at higher concentration it is
bactericidal
cationic CHX molecule+ negatively charged bacterial cell wall
Instant adsorption of CHX to Phosphate containing
compounds
CHX binds with the phospholipids in the inner cell membrane
causing cell wall integrity
Leakage of the lesser molecular weight components viz. potassium
ions
[This is the sub lethal stage of CHX. The action can be reversed. This
marks the bacteriostatic property of CHX. If the conc. Is increased and
the action continues, the CHX becomes bactericidal in nature]](https://image.slidesharecdn.com/chlorhexidine-130124014926-phpapp01/85/Chlorhexidine-5-320.jpg)
![Intracellular coagulation
Slows down leakage of intracellular
components
Cytoplasmic coagulation
Irreversible cell damage [bactericidal]](https://image.slidesharecdn.com/chlorhexidine-130124014926-phpapp01/85/Chlorhexidine-6-320.jpg)






![Chlorhexidine products:
Mouth rinse- aqueous/ alcohol solutions of
0.2% [Peridex, Periogard, Periosol]
Gel [corsodyl dental gel]
Sprays [Hibispray]
Tooth pastes
Varnishes
Chewing gums
Periodontal dressings
Subgingival plaque control [Periochip]](https://image.slidesharecdn.com/chlorhexidine-130124014926-phpapp01/85/Chlorhexidine-13-320.jpg)
